X. Jones Reaction: The Oxidation of Borneol to Camphor
Oxidation and reduction reactions are two very important classes of reactions in organic chemistry. Oxidation reactions are those accompanied by a loss of electrons and a gaining in oxidation number. Reduction reactions result from a gaining of electrons and the subsequent reduction in oxidation number.
While those theoretical definitions of oxidation and reduction hold true in organic chemistry, a more qualitative way of determining oxidation and reduction reactions has been developed.
Oxidation reactions are ones in which:
a) oxygen is inserted into a bond
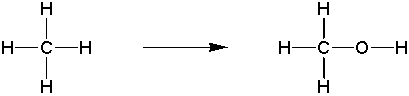
b) hydrogen is lost across a bond
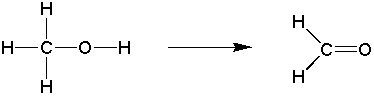
c) a more electronegative atom replaces a more electropositive one.
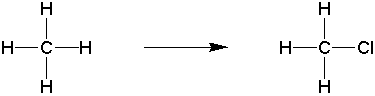
Thus, reactions can be classified as oxidation reactions based on inspection.
A very important group of oxidation reactions uses various forms of chromium as the oxidizing agent. Reactions of this type are called Jones oxidations. The common chromium reagents in use are CrO3/ H+; H2CrO4; Cr2 O72-/ H+. All of these reagents have chromium in the +6 oxidation state and are called Jones reagents.
The result of carrying out a Jones oxidation is that
a) primary alcohols oxidize to form carboxylic acids
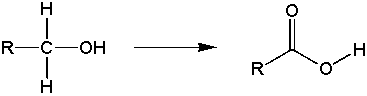
b) secondary alcohols oxidize to form ketones
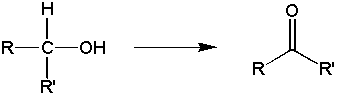
c) tertiary alcohols do not undergo Jones oxidations
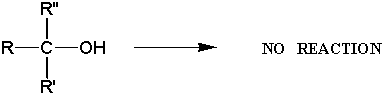
d) aldehydes oxidize to form carboxylic acids
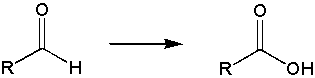
and the Cr6+ is reduced to Cr4+.
A more thorough review of oxidation reactions will be covered by your recitation instructor. You may also wish to review Chapter 8 of the Vollhard and Schore text.
Goal: The goal of this lab is to oxidize borneol to camphor using
the Jones reagent.
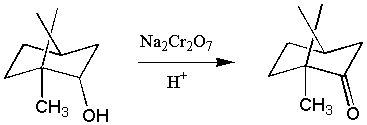
You will then purify the product using sublimation and characterize your product by melting point and IR spectroscopy.
Procedure:
CAUTION: WE WILL BE WORKING WITH CONCENTRATED SULFURIC ACID, BE VERY CAREFUL. PROTECTIVE RUBBER GLOVES ARE RECOMMENDED WHEN HANDLING THE SULFURIC ACID.
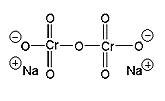
1. First prepare the Jones reagent: dissolve 2.0 g of sodium dichromate dihydrate in 6 mL of water in a 250 mL Erlenmeyer flask. Use a graduated cylinder to measure out approximately 2 mL of concentrated sulfuric acid from a supply that the lab instructor will provide for you. Slowly add the sulfuric acid to the Erlenmeyer flask. Much heat is generated. Be careful!
2. Add approximately 6 mL of water to the dichromate solution and then transfer the diluted solution to a 50 mL Erlenmeyer flask and cool in an ice bath.
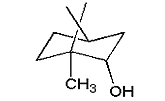
3. Dissolve 1.0 g of d,l-borneol in 4 mL of diethyl ether and cool on ice. Using a pipette, slowly add the cold dichromate solution to the borneol solution, stopping every few drops to swirl the mixture. Again, much heat is generated and you don't want the temperature of the solution to rise rapidly. Add the dichromate slowly. Continue swirling for about 5 minutes after all the dichromate is added.
4. Pour the reaction mixture into the 125 mL separatory funnel and add 30 mL each of ether and water. Shake and vent. Reread Chapter 37 in the OCLSM for a review of extraction. The layers that form may be very hard to distinguish, as in the image below.

You might need to use a flashlight to see a line of separation as shown in the picture below. When in doubt, check with your lab instructor. Keep both layers of this extraction.

5. Return the aqueous portion to the separatory funnel and extract twice more with 10 mL portions of ether. Combine all of the ether extracts.
6. Return the ether layers to the separatory funnel and wash with two 10 mL portions of 5% sodium bicarbonate to remove unreacted chromic acid. Vent immediately as pressure will build up quickly.
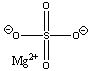
7. Dry the organic layer with magnesium sulfate. See Chapter 10 of the OCLSM or the web material for more information about drying agents.
8. Remove the magnesium sulfate using vacuum filtration. Keep the ether layer in the filter flask and add a boiling chip. Evaporate the ether on a gentle steam bath until a foamy residue remains.
9. Carry out a sublimation as described on page 212 of the OCLSM. Your instructor will give you precise instructions on how to do this procedure including the use of a vacuum trap. A picture of this setup is given below.

Note:Your cold fingers must be dry in order for the sublimation to be successful.
10. After sublimation is complete, slowly remove the cold finger. The product should have formed on the outside surface, as indicated in the following picture.

Scrape the camphor off of the test tube onto a piece of filter paper. Weigh your product to determine the final yield.
11. Determine the melting point of your camphor.
12. Run an IR of your product to assist you in its final characterization. You may wish to compare it to the IR spectrum of borneol. Also for reference, you might want to check the IR of mineral oil.
13. Calculate the percent yield for the reaction.
Chemical Info.
|
|
2-D structure |
3-D structure |
More Chem. Info. |
|
borneol |
|
||
|
sodium dichromate |
|
|
|
|
sodium bicarbonate |
|
|
|
| sulfuric acid |  |
|
Link to MSDS |
|
ether |
|
||
|
magnesium sulfate |
|
|
Last updated: 07-31-04