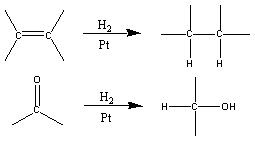
Reduction reactions are one of the major classes of reactions in organic chemistry. The opposite of oxidation (discussed earlier), reduction reactions can be determined by looking for:
a) a loss of oxygen from a bond
b) the addition of hydrogen to a bond
c) the replacement of a more electronegative atom with a less electronegative atom.
There are many methods of reduction in use today. A general
method of reduction is hydrogenation which reduces most types of multiple
bonds.

A more specific reduction method involves hydride (H-)
transfer reagents. Here only carbonyl groups are reduced.
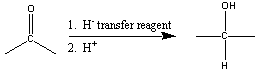
Further information on the various types of reduction reactions and their mechanisms will be discussed in class. Section 8.6 of Vollhardt and Schore also covers this topic.
Goal: The goal of this lab is to convert benzophenone to diphenylmethanol
using sodium borohydride as a reducing agent.
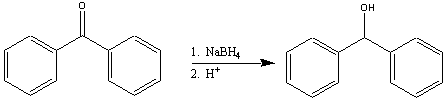
You will isolate the solid product and purify it by recrystallization. You will then characterize it using the techniques you have mastered over the term.
Procedure:
1. Set up a dry reflux apparatus as seen on pages 202-203 of the OCLSM. You should also read Chapter 23 of the OCLSM for more information on reflux.
2. Check your drying tube. Make sure that the calcium chloride inside is free-moving. The drying tube is good if you can hear the beads rattling around inside. If you cannot hear rattling, replace the drying agent (calcium chloride) using the method your instructor will demonstrate.
3. Weigh 1.0 g of sodium borohydride into a covered 50 mL beaker. Sodium borohydride is highly caustic and must not come in contact with your skin, eyes, or nasal passages.
4. In a 100 mL round bottom flask dissolve 5.5 g of benzophenone in 50 mL of methanol and cool the solution on ice. Add boiling chips to the flask.
5. With the flask on ice, slowly and carefully add the sodium borohydride to the benzophenone over a period of ten minutes. Wait until vigorous evolution of hydrogen has stopped before moving on to the next step.
6. Connect the round bottom flask to the reflux apparatus and reflux on a steam bath for 20-30 minutes. Cool the mixture on ice. Slowly add, through the condenser, enough 6M HCl to acidify the solution. What do you think the precipitate is? Check the pH with litmus paper (red=acidic).
7. Transfer the solution to a 500 mL separatory funnel and extract 2X with 40-50 mL portions of ether.
Note: Some boric acid will precipitate upon addition of ether. Add about 50 mL of water to dissolve the H3BO3 and allow the layers to form. Combine the ether layers.
8. Wash the ether extracts with 50 mL of water and dry the ether with magnesium sulfate.
9. Filter off the drying agent and evaporate the ether on a steam bath.
10. Recrystallize your product using hexanes. Note: Hexanes have a boiling point of just a few degrees higher than the product's melting point. Is this a problem? Hint: Find the literature value of the melting point of diphenylmethanol and then review the critieria for a suitable recrystallization solvent.
Note: If you know the melting point of diphenylmethanol (as you should), then you also recognize one potential problem with recrystallizing from hexanes. Talk to your TA about how to solve this potential problem.
11. Weigh your recrystallized product to determine the % yield of the reduction.
12. Take a melting point and an IR of your final product to aid in its
characterization. Compare the IR of the product to the IR spectrum of benzophenone
and the IR of mineral oil. What absorptions should/should not appear
in the product's IR spectrum?
Chemical Info.
| 2-D structure | 3-D structure | More Chem. Info. | |
| benzophenone | 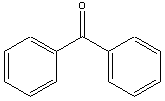 |
click here for 3-D view | Link to MSDS |
| diphenylmethanol | 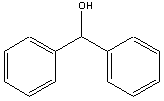 |
click here for 3-D view | Link to MSDS |
| sodium bicarbonate | 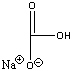 |
Link to MSDS | |
| methanol | 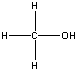 |
click here for 3-D view | Link to MSDS |
| hydrochloric acid | Link to MSDS | ||
| ether | 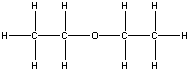 |
click here for 3-D view | Link to MSDS |
| magnesium sulfate | 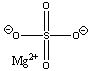 |
Link to MSDS | |
| hexanes | Link to MSDS |
Last updated: 07-31-04