IX.
The Synthesis of Alkenes: The Dehydration of Cyclohexanol.
Elimination reactions, in organic chemistry, are extensively used to synthesize alkenes. The two most common routes for this type of reaction are the E1 and E2 mechanisms. Several factors determine the pathway that occurs including type of substrate (1E, 2E, 3E), reaction conditions (temperature, solvent), nature of the base (!OH, H2O) and nature of the leaving group. The following examples illustrate these effects.
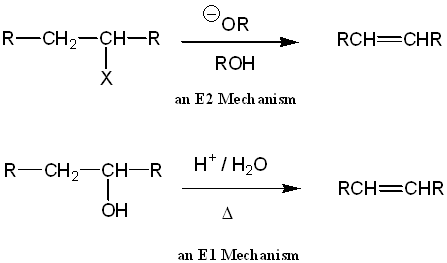
When the leaving group is a water molecule, the elimination is known as a dehydration reaction, as shown below.
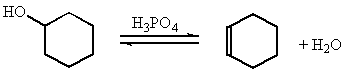
Goal: The
goal of this week’s lab is to synthesize cyclohexene from cyclohexanol using
the method of dehydration. You will
then test for the presence of the carbon-carbon double bond by the addition of aqueous
bromine and characterize your product using IR.
Procedure:
Note: Cyclohexene is a malodorous liquid. To avoid filling the lab with the odor of cyclohexene, work in the hood at all times. Wash all glassware that touches cyclohexene with acetone in the hood and then clean with soap and water. When you go to obtain an IR of your product, keep it contained in a stoppered flask.
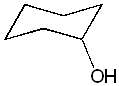
1. Place 10 mL of cyclohexanol (pictured below) in a 100-mL round-bottom flask. Add 12 mL of 85% phosphoric acid and several boiling chips. Swirl the flask to mix the layers.

Cyclohexane Liquid
2.
Attach the flask to a simple distillation apparatus (p.
166 of OCLSM) using a 50-mL round-bottom flask immersed in an ice-water bath
as the receiving flask.
3.
Heat the mixture so that cyclohexene being synthesized begins to slowly
distill. You will need to turn up
the heating mantle control to 55% (55 or 70). The
vapor temperature should not be allowed to exceed 100-150ºC;
record the actual temperature at which the liquid distills.
Continue distilling the mixture until approximately 5 to 10 mL of liquid
remains in the distillation flask.

Distillation
apparatus
4.
Transfer the material in the receiver flask (pictured below) to a small
separatory funnel and wash with one 5-mL portion of 10% sodium carbonate
solution (VENT!), then with the two
10-mL portions of the saturated NaCl solution.

5.
Transfer the organic liquid to a 25-mL Erlenmeyer flask and dry over
anhydrous sodium sulfate.
6. Decant the liquid into a clean, pre-weighed flask and obtain a yield.
7.
Test your product for the presence of an alkene with the bromine test
(pictured below).
Add a small amount of your product to a test tube.
Then add a few drops of dilute bromine solution.
What happens? What does this
result tell you?

Bromine
(orange) Added to Alkene in Test Tube
8.
Obtain an IR of your product, and compare this to the IR of the starting
material.
|
|
2-D structure |
More Chem. Info. |
|
cyclohexanol |
|
|
|
bromine |
Br-Br |
|
|
sodium bicarbonate |
|
|
| phosphoric acid | 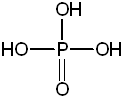 |
Link to MSDS |
|
magnesium sulfate |
|
Last updated: 10-21-05