





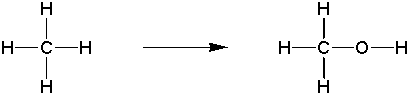
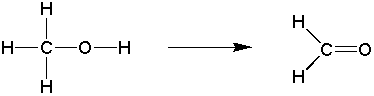
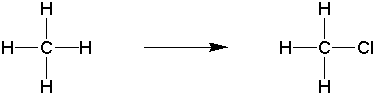
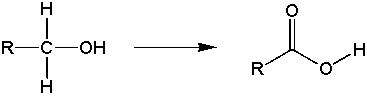
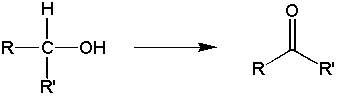
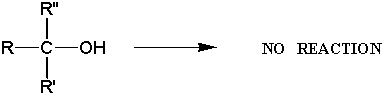
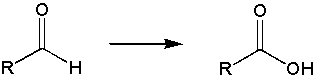
Goal: The goal of this lab is to oxidize borneol to camphor using the Jones reagent. You will then purify the product using sublimation and characterize your product by melting point and IR spectroscopy.
Procedure:
1. Transfer glacial acetic acid (3.0 mL) to a 25 mL Erlenmeyer flask equipped with a stirring bar.
2. Transfer borneol (0.500 g) to the 25 mL Erlenmeyer flask. Measure the mass of borneol in a weighing boat. If the borneol is in clumps, carefully break the clumps into smaller pieces with a stirring rod. Powder dissolves more readily.
3. Clamp the Erlenmeyer flask with the borneol/acetic acid mixture to the monkey bars over a magnetic stirrer. Stir gently until the borneol dissolves (~ 3 minutes).
4. After the borneol dissolves, place an ice bath under the reaction flask. Prepare the ice bath from a mixture of ice water. Lower the reaction flask into the ice bath. The glacial acetic acid will begin to solidify. The glacial acetic acid will liquefy as the sodium hypochlorite is added in the next step.
5. Add sodium hypochlorite (~4.0 mL) dropwise with a pipette over 5 minutes. You will notice the reaction mixture turning yellow as Cl2(g) evolves in the reaction.
6. Stir the reaction mixture on ice for 30 minutes. Keep the reaction flask on ice throughout the reaction to keep the temperature below 25°. Watch for the formation of camphor which is a white solid.
7. Hypochlorous acid forms in the reaction and must be present throughout the reaction. So, during the reaction, test for the presence of hypochlorous acid (HOCl) with KI-starch paper . In the hood, place the tip of a glass stirring rod into the reaction mixture. Transfer a drop of the reaction mixture to the KI-starch paper. If the HOCl is present the KI-starch paper will turn blue/black. If the KI-starch paper test for HOCl is negative, add 1.00 mL of sodium hypochlorite dropwise with a pipette. Stir 2 minutes and repeat the test. Check for HOCl every ten minutes until the reaction is complete.
8. After 30 minutes, neutralize the remaining HOCl with a saturated solution of sodium bisulfite. Introduce a few drops of saturated sodium bisulfite, NaHSO3(aq), while the reaction mixture stirs on ice. Test for HOCl with the KI starch paper after five drops of NaHSO3(aq) has been added. Less than 10 drops of NaHSO3(aq) should be required.
9. Place a Buchner filter equipped with a filter paper into the vacuum flask. Use a pipette to wet the filter paper with small amounts of water before filtering. Activate the vacuum by turning on the water at the aspirator. Filter the solid camphor from the reaction mixture. Transfer the solid camphor from the solid camphor from the reaction mixture to the Buchner funnel. During filtration keep the camphor in the center of the filter paper to avoid loss. Rinse the reaction flask with 15 mL of cold water to recover residual camphor. Transfer the rinse to the Buchner funnel. Repeat.
10. Dry the product for five minutes by vacuum filtration.
11. While the product is drying, begin setting up the sublimation apparatus by obtaining a 1 L filter flask, a stopper with a hollow glass rod, and a cold finger. Also ensure that the steam lines do not have condensed water in them as you will need to set up a steam bath.
a. On the bench in the hood, clamp a 1 L vacuum filter flask to the monkey bars with a three prong clamp.
b. Connect heavy red tubing to the hollow glass rod in the rubber stopper.
c. Place the stopper with the hollow glass rod into the vacuum flask.
d. Connect the red tubing to the aspirator.
e. Connect another pieceof heavy red tubing to the side arm of the 1 L vacuum flask. Then use that read tubing to connect the 1 L filter flask (vacuum trap) to a 125 mL vacuum flask.
f. Clamp the 125 mL vacuum flask to the monkey bars. Leave some space for the steam bath which will be placed under the 125 mL vacuum flask.
g. Transfer your dried product to the 125 mL vacuum flask.
h. Insert cold finger into the 125 mL vacuum flask. Check the depth of the cold finger. The bottom of the test tube (that serves as the cold finger) should be ~1.0 inch from the camphor in the bottom of the flask. If the test tube needs to be adjusted, remove it from the flask before adjusting the test tube. Very carefully, adjust the stopper around the test tube. Return the test tube to the flask to check the adjusted depth. Never force the test tube through the stopper. Never adjust the test tube while the cold finger is in the filter flask. Consult your TA if you have difficulty.
i. Ensure that the stopper fits in the vacuum flask properly. If the stopper falls too far into the flask initially, it might be pulled into the flask under vacuum. Consult your TA if you are unsure.
j. Once the cold finger is secure in the vacuum flask, move the steam bath under the vacuum flask. Connect the steam bath to the steam outlet. Place the drain hose from the steam bath into the sink. Do not activate the steam.
k. Slowly activate the vacuum. If you activate the vacuum too quickly some camphor might be lost.
12. Place paper towels around the stopper of the cold finger. Add some ice to the cold finger. Avoid allowing ice to fall into the stopper where it connects to the cold finger and to the flask. Change the paper towel as necessary. Keep the top of the cold finger wrapped in a paper towel as you activate the steam. Sublimation will begin to occur in 5-10 minutes.
13. When the sublimation is complete, shut off the steam and pipette water from the cold finger. Carefully unclamp the filter flask and dump the ice from the cold finger. Clamp the flask to the monkey bars. Shut off the vacuum at the aspirator. Disconnect the red tubing that connect the vacuum trap to the aspirator. Breaking the vacuum too abruptly at 125 mL Erlenmeyer flask might knock sublimed camphor from the cold finger.
14. Carefully remove the cold finger from the vacuum flask. Scrape the purified camphor from the cold finger into a tared weighing boat. Calculate % yield.
15. Collect IR and determine mp.
| Chemical Information | |
| Name | Structure (2-D) |
| borneol | 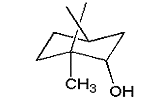 |
| glacial acetic acid | 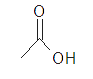 |
| sodium hypochlorite | 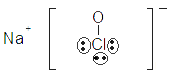 |
| hypochlorous acid | |
| sodium bisulfite | 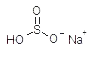 |