

1. Theoretical definition of reduction
a) gain of electrons
b) reducing of oxidation number
i) Cr+6 + 2e- -> Cr+4
2. Opposite of oxidation
3. Typical reducing reagents
a) catalytic hydrogenation
i) H2/Pt
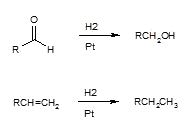
a) high temperature
b) high pressure
c) not selective (C=0 and C=C)
b) Hydride (H-) Transfer Reageants
i) NaBH4

a) selective - only
b) mild
c) reduces aldehydes to 1° alcohols and ketones to 2° alcohols
d) four moles of H- per mole of BH4-
2) LiAlH4
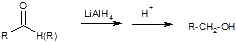
a) stronger
b) reduces acids, esters and aldehydes to 1° alcohols, ketones to 2° alcohols.
c) explosive with H2O
d) 4 moles of H-/mol AlH4-
3) LiAl(tBuO)3H
3H.gif)
a) selective for acid chlorides to aldehydes
i) single H-
ii) low temperature
iii) steric hinderance
4) All are selective for the C=O group and will not reduce to C=C
5) The hydride transfer mechanism
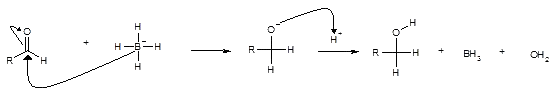
Note: The H2 produced from the above reaction is not the H2 the students observe upon addition of NaBH4





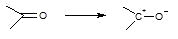
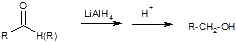
3H.gif)
