





Goal: The goal of this lab is to separate benzoic acid from a mixture containing benzoic acid, cellulose (a natural polymer of glucose) and methyl orange (a common acid/base indicator). You will use a chemically active extraction to convert the water insoluble benzoic acid into its water soluble salt by treating the carboxylic acid with base. Finally the benzoic acid will be precipitated by adding strong acid to the carboxylate salt solution. The following extraction schemes show the methodology you will use.

PROCEDURE:
1) Weigh 4 g of the crude benzoic acid mixture using the analytical
balance and place it in a 125 mL Erlenmeyer flask. Proper weighing
procedure is covered on pages 55-56 of the OCLSM.
Note: We do not use vials in the lab so a paper towel
or watch glass will have to be substituted.
2) Add 50 mL of diethyl ether to the flask. See page 3 in the OCLSM for proper procedures when dealing with the transfer of volatile liquids and use of the hood.
3) Add boiling stones (page 144 in the OCLSM) and heat the mixture on a steam bath until the ether begins
to boil. Proper use of a steam bath is found on page 147 of the
OCLSM.
4) Vacuum filter the mixture using a Buchner funnel and a filter flask (page 109 in the OCLSM). Note: The Buchner funnel may be used to separate any solid from a liquid. It is not just for the separation of crystals. Observations: Which components remain in the ether? Which have been filtered out? Why?
5) Place the filtrate (ether) in a 500 mL separatory funnel and extract
twice with two 30 mL portions of 1 M sodium hydroxide (NaOH). Collect
and keep the aqueous layers from both extractions. Combine
the two aqueous layers in a 250 mL beaker or a flask. Chapter
15 of the OCLSM covers this topic in great detail.
Formation of two layers after the addition of NaOH to ether
How to shake the separatory funnel
Venting process
Collection of aqueous layers
6) Cool the combined aqueous extracts on ice. Acidify with 50
mL of 6M HCl. Cool on ice again.
Addition of HCl to the aqueous layers. Observations: During acidification the solution
should turn a bright pink. Why?
7) Collect the benzoic acid by suction filtration and save it on a watch
glass. You will recrystallize it during the next laboratory period.
Observation: Comment on the color, shape
and quantity of your benzoic acid.
| CHEMICAL INFORMATION | |
| Name | Structure (2-D) |
| benzoic acid | 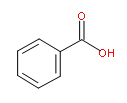 |
| cellulose | 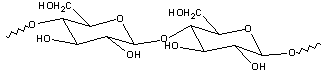 |
| methyl orange | 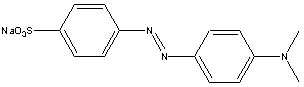 |
| diethyl ether |  |