


Goal: The goal of this experiment is to separate a spinach extract into some of its individual components using column chromatography. You will also perform some analytical calculations on the separated molecules using TLC.
Procedure: Column Chromatography
Note: You should carry out this experiment in groups of 3
or 4. The following method of packing a column is called dry
packing. Sometimes it is preferred to slurry pack a column.
Your instructor will inform you as to the method you are to use.
1. First prepare your column as follows: Push a small plug (about
1/4 of an inch thick) of cotton to the bottom of the column with a
glass rod. NOTE: Too much cotton will cause the column
to run too slowly. Next fill the column about one-half
to three-quarters full with either ligroin or petroleum ether. Through a dry funnel,
introduce enough sand to form a 1 cm layer over the cotton and level
the surface of the sand by tapping the column gently. Through a funnel
add slowly, with tapping, 11 g of alumina to the column. When most
of the alumina has settled, wash down any alumina that may be adhering
to the walls of the column with additional ligroin or petroleum ether. Make sure that
the alumina forms a nice, smooth column and that it contains no bubbles
or channels. (These can sometimes be removed by gently tapping the
column with a piece of rubber tubing.) When all of the alumina has
settled, add a little more sand to form an even protective layer on top.
Finally, open the stopcock of the column and permit the solvent to pass
through the column into a beaker, leaving a small amount of solvent
above the sand layer. NEVER LET A COLUMN RUN DRY! Your
column is now ready to use.
2. Prepare 50 mL of 9:1 ligroin/acetone.
3. Open the stopcock and allow the liquid to become level with the upper
layer of sand. Now your instructor will add about 2mL of the spinach
pigment to your column. Load the spinach pigment onto the column
by opening the stopcock. You should stop when the spinach pigment is
level with the sand. Add 3-5 mL of (9:1)
ligroin/acetone, and once again open the stopcock until the solution is
just above the sand. This will cause the spinach extract to concentrate
on the alumina layer. Now you are ready to elute the column.
4. Elute the column with the 9:1 ligroin:acetone solution to “wash out”
the first fraction. Add enough eluent to move the orange-yellow carotenes
and gray pheophytins through the column. Collect this fraction in
a 50 ml beaker. Collect only the colored portion of the eluant
in this beaker. The rest of the eluant can be collected in other
glassware and disposed of according to your instructor.
5. Prepare 50 mL of a (1:1) mixture of acetone:ligroin and elute the
column with this mixture. Collect the green eluent in a 50 mL beaker.
This should remove the various chlorophyll molecules from the spinach pigment.
6. Finally elute the column with absolute methanol to remove the most
polar plant pigments from the column. Collect these in a clean 50mL
beaker.
7. DO NOT DISCARD ANY OF YOUR FRACTIONS!
8. Consult with your instructor for the proper procedures for cleaning
up your column.
Procedure: Thin Layer Chromatography
1. Concentrate the three fractions from your column experiment.
Add a boiling chip to each and evaporate the solvent on a gentle
steam bath.
2. Add one or two drops of 3% 1-butanol in ligroin to each fraction
to form a concentrated sample.
3. Obtain a precut silica gel TLC plate from your instructor.
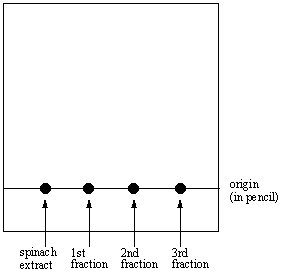
4. Using a pencil draw a line approximately 1/4 inch from the
bottom of the plate. This will be called the origin.
5. Use a capillary spotter (page 223, OCLSM) to apply each of your fractions and the original
spinach extract to the plates at the origin. Repeated applications
of the analytes may be necessary. Your plate should look like this:
6. Develop your plate using the recommended solvent system. See page 226 of the OCLSM for the construction
of a developing chamber. You will need to use a 400 mL
beaker as a chamber.
7. After development is complete, calculate the Rf values for
your fractions and the original spinach sample.
| Chemical Information | |
| Name | Structure (2-D) |
| ligroine | |
| acetone | 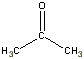 |
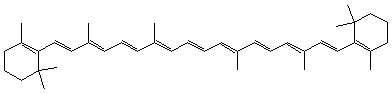
| carotene | see below |
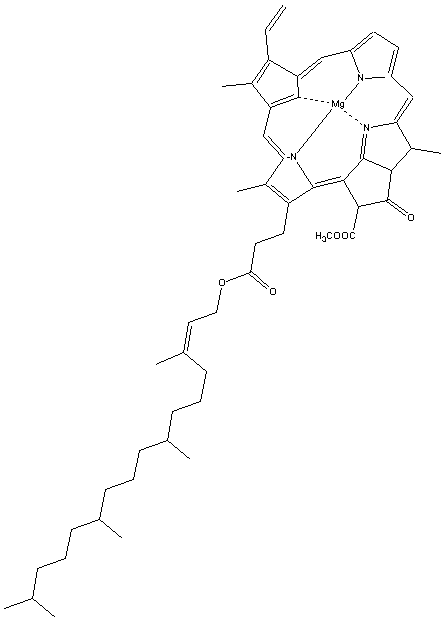
| chlorophyll-a | see below |
| methanol | 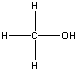 |
| alumina | |
| 1-butanol | 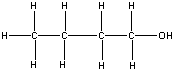 |