


Goal: The goal of this lab is to separate the two components of a binary mixture using fractional distillation. A plot of volume of distillate (mL) vs. temperature (ºC) will be drawn. This graph will then be used to identify the two components of the mixture and to determine the relative amounts of each component (% composition) in the mixture.
Procedure:
1. Construct the fractional distillation apparatus shown on page 180 of the OCLSM. Use a 10 mL graduated
cylinder as a receiving vessel.
2. Add 50 mL of an unknown obtained from your instructor to the
distilling flask. Be certain to add a few boiling chips to the
flask. Also record your unknown letter.
3. Heat the flask in a heating mantle with a variac setting
between 50 and 60.
4. As the distillate begins to collect in the graduated cylinder
begin to take data. You want to record the distillate temperature
for each mL of distillate collected.
5. If the rate of distillation is faster than ~ 1 drop/s you may want
to lower the variac setting. It is difficult to obtain a satisfactory
separation of the mixture using the short fractionating column you are
using. Separation can be achieved by carefully controlling the
temperature of the pot and the distillation rate.
6. After the first component has been removed, the temperature should
rise rapidly. This is the second component starting to distill.
Continue recording data as you distill the second component.
7. If the temperature drops after the first component is removed
you may need to increase the variac to ~75. Consult with your
instructor if this situation arises.
8. When you have a few mLs left in the distillation flask turn off the
variac. Allow the apparatus to cool, then take it apart. Your
distillation is completed.
9. Plot a graph of mL of distillate (x-axis) vs. temperature (y-axis).
Use this graph to determine the % composition of your original sample
and to identify the two components of your original sample using the data
shown below.
10. Have a great day distilling!
| FRACTIONAL DISTILLATION UNKNOWNS | ||
| Compound | Boiling Point ºC | Structure (2-D) |
| Acetone | 56.5 | 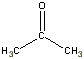 |
| Methanol | 65.0 | 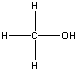 |
| Ethanol | 78.5 | 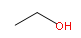 |
| Isopropanol | 82.4 | 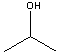 |
| Isopropyl acetate | 88.8 | 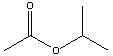 |
| Water | 100.0 | |
| Toluene | 110.6 | 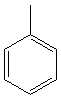 |
| Methyl isobutyl ketone | 116.9 | 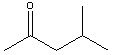 |
| 2-Methoxyethanol | 124.0 | 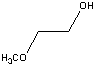 |