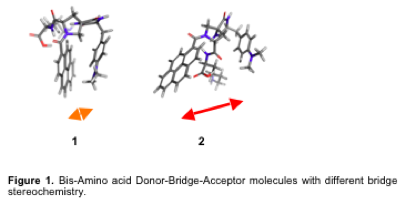
Water-mediated Electron Tunneling Studies
Group members: Prasun Mukherjee, Daniel N. Lamont, Matthew Parker
Collaborators: Professor Christian Schafmeister (Temple University).
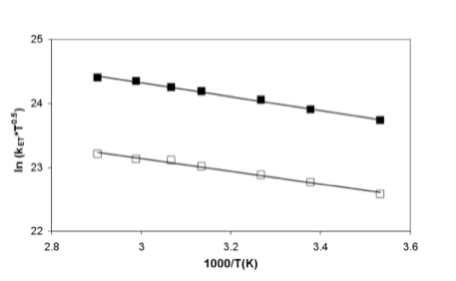
This difference in geometry also provides two different “line-of-sight” donor to acceptor distances. This will allow us to probe the participation of water molecule(s) in electron transfer from donor to acceptor in these systems. Our study has shown that the electron transfer (ET) rate is much faster in 1 compared to 2 (Figure 2). This shows that electron transfer happens through space instead of through the bridge in these molecules. The electron transfer rate in 1 is higher because of the active participation of the hydration layer formed between donor and acceptor in 1, whereas in 2, the water molecules behave like bulk water.
Water molecules influence electron transport in biomolecules and play a key role in biologically vital processes in living cells. We investigate the role of water molecules in electron transfer reactions by studying the photoinduced electron transfer rate in two Donor-Bridge-Acceptor (DBA) bis-amino acid oligomers that contain a keto pyrene group as an acceptor unit and dimethylaniline (DMA) as a donor unit in water. The DBA molecules differ by their bridge stereochemistry (Figure 1). Molecule 1 has a cleft between the donor and acceptor whereas molecule 2 does not form any well-defined cleft.