HPS 1702 Junior/Senior Seminar for HPS Majors
HPS 1703 Writing Workshop for HPS Majors
Spring term 2014
Repeatability and Reproducibility of Experiment
Back to course document list.
Science, December, 2011
Infection and Immunity, December, 2010
Journal of IR




From IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997).
(International Union of Pure and Applied Chemistry)

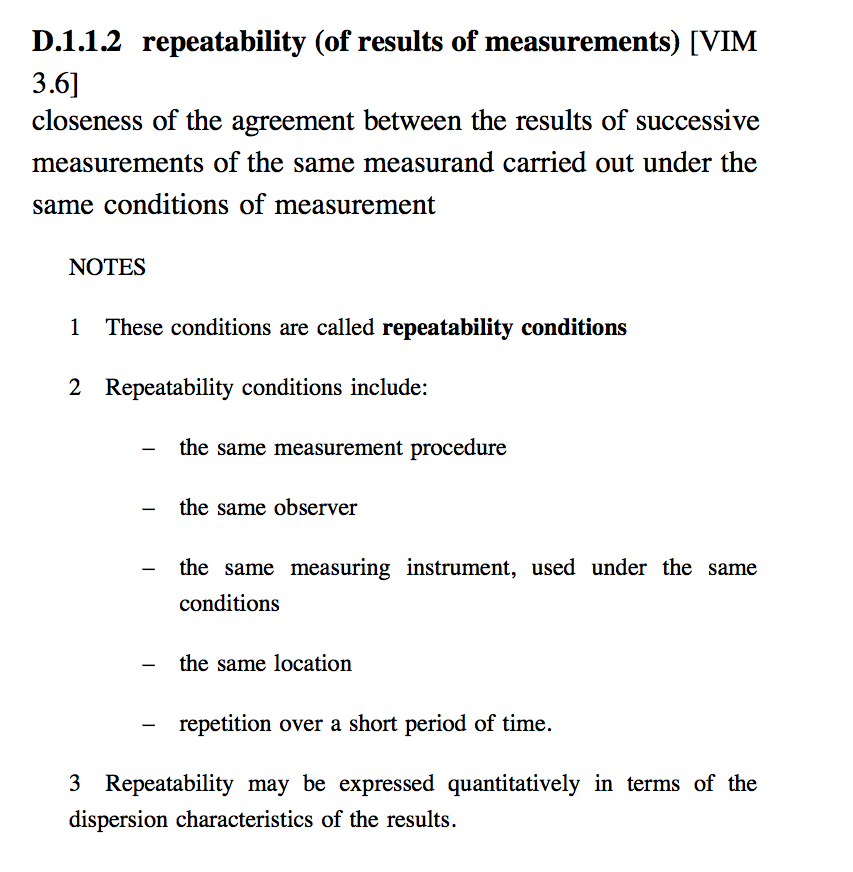
repeatability
The closeness of agreement between independent results obtained with the same method on identical test material, under the same conditions (same operator, same apparatus, same laboratory and after short intervals of time).
The measure of repeatability is the standard deviation qualified with the term: `repeatability' as repeatability standard deviation.
In some contexts repeatability may be defined as the value below which the absolute difference between two single test results obtained under the above conditions, may be expected to lie with a specified probability.

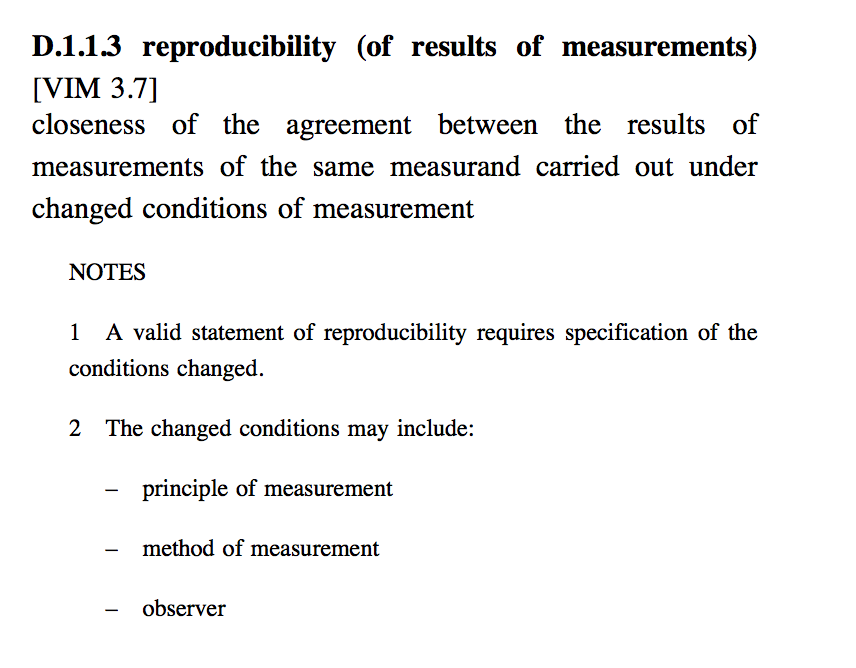
reproducibility
The closeness of agreement between independent results obtained with the same method on identical test material but under different conditions (different operators, different apparatus, different laboratories and/or after different intervals of time).
The measure of reproducibility is the standard deviation qualified with the term 'reproducibility' as reproducibility standard deviation.
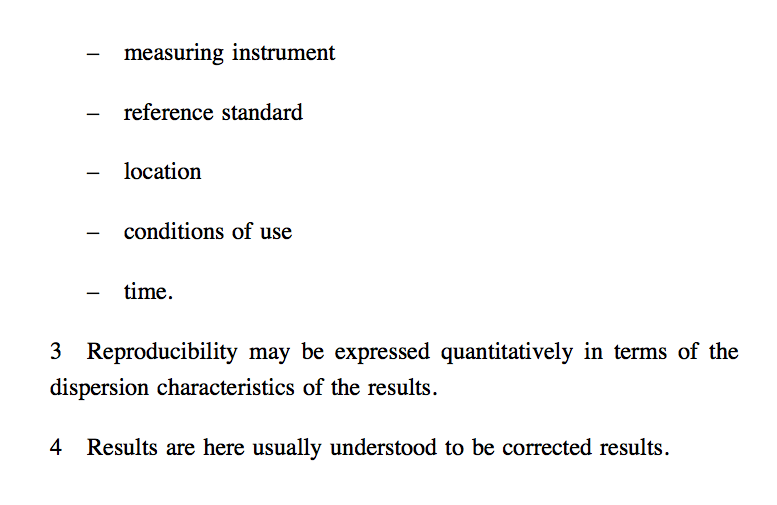
In some contexts reproducibility may be defined as the value below which the absolute difference between two single test results on identical material obtained under the above conditions, may be expected to lie with a specified probability. Note that a complete statement of reproducibility requires specification of the experimental conditions which differ.

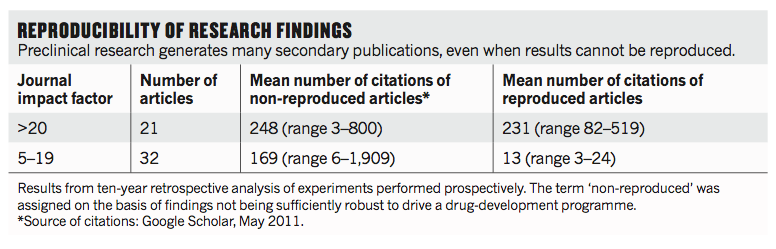
Over the past decade, before pursuing a particular line of research, scientists (including C.G.B.) in the haematology and oncology department at the biotechnology firm Amgen in Thousand Oaks, California, tried to confirm published findings related to that work. Fifty-three papers were deemed 'landmark' studies (see 'Reproducibility of research findings'). It was acknowledged from the outset that some of the data might not hold up, because papers were deliberately selected that described something completely new, such as fresh approaches to targeting cancers or alternative clinical uses for existing therapeutics. Nevertheless, scientific findings were confirmed in only 6 (11%) cases. Even knowing the limitations of preclinical research, this was a shocking result.
...
...
Unfortunately, Amgen's findings are consistent with those of others in industry. A team at Bayer HealthCare in Germany last year reported4 that only about 25% of published preclinical studies could be validated to the point at which projects could continue. Notably, published cancer research represented 70% of the studies analysed in that report, some of which might overlap with the 53 papers examined at Amgen.