
Department of Molecular Genetics and Biochemistry
University of Pittsburgh School of Medicine
Pittsburgh, PA 15261
| The Std1 protein modulates the expression of glucose-regulated genes but its exact molecular role in this process is unclear. A two-hybrid screen for Std1-interacting proteins identified the hydrophilic C-terminal domains of the glucose sensors, Snf3 and Rgt2. The homologue of Std1, Mth1 behaves differently from Std1 in this assay by interacting with Snf3 but not Rgt2. Genetic interactions between STD1, MTH1, SNF3 and RGT2, suggest that the glucose signaling is mediated, at least in part, through interactions of the products of these four genes. Mutations in MTH1 can suppress the raffinose growth defect of a snf3 mutant as well as the glucose fermentation defect present in cells lacking both glucose sensors (snf3 rgt2). Genetic suppression by mutations in MTH1 is likely to be due to the increased and unregulated expression of hexose transporter genes. In media lacking glucose or with low levels of glucose, the hexose transporter genes are subject to repression by a mechanism that requires the Std1 and Mth1 proteins. An additional mechanism for glucose sensing must exist since a strain lacking all four genes (snf3 rgt2 std1 mth1) is still able to regulate SUC2 gene expression in response to changes in glucose concentration. Finally, studies with GFP fusion proteins indicate that Std1 is localized to the cell periphery and the cell nucleus, supporting the idea that it may transduce signals from the plasma membrane to the nucleus. |
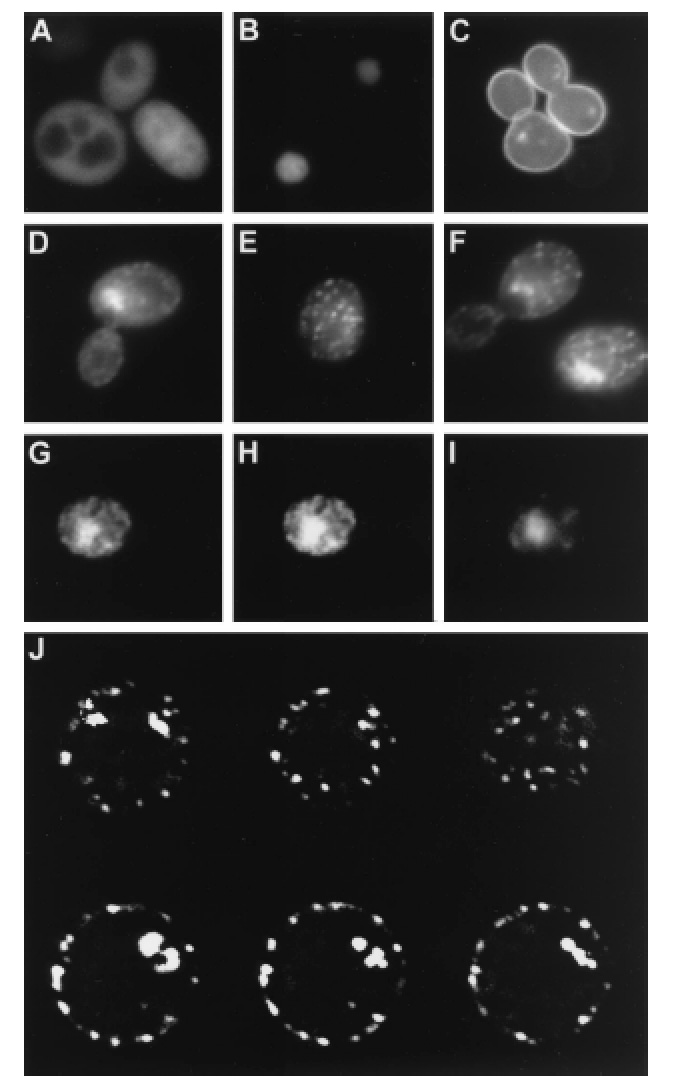
Std1 is localized in cell nucleus and at the plasma membrane.
Wild-type (A to E) or snf3 rgt2 (F) cells were analyzed by
fluorescence microscopy. Cells expressed either unfused GFP from the
GAL1 promoter (A), histone-GFP fusion (B), Snf3-GFP fusion (C),
or Std1-GFP fusion (D to F). Fluorescence images were collected of a single
Std1-GFP-expressing cell (G to I) that had been fixed with formaldehyde
and stained with Hoechst dye. (G) Std1-GFP fluorescence; (I) Hoechst dye
fluorescence; (H) composite image of both showing colocalization of the
Hoechst and GFP fluorescence. (J) A single Std1-GFP-expressing cell that
showed both nuclear and punctate cytoplasmic fluorescence was analyzed
sequentially at six different focal planes.