Hemodialysis Home End Stage Renal Disease
(ESRD) Principles
Dialyzers Modeling
Hemodialysis Therapy Demonstration History
Principles
of Hemodialysis
Kidney function in patients with
partial or complete renal failure is insufficient to adequately remove excess
electrolytes (salts) and waste metabolites generated through ordinary
metabolism. As a result, the
concentrations of these species will build in the body to toxic levels unless
something is done to help remove them.
 The
major source of waste metabolites is the liver since this is where most of the
energy conversion processes in the body take place. The end products of carbohydrate and fat metabolism tend to be
water and carbon dioxide, both of which can be lost from the body through
respiratory processes (breathing). The
end products of protein metabolism, however, are generally eliminated through
the kidneys. Urea is the largest mass
of waste metabolite produced in the liver from protein metabolism and is
generally used as a marker of renal function because it is present in large
quantities in the blood and is easily measured.
The
major source of waste metabolites is the liver since this is where most of the
energy conversion processes in the body take place. The end products of carbohydrate and fat metabolism tend to be
water and carbon dioxide, both of which can be lost from the body through
respiratory processes (breathing). The
end products of protein metabolism, however, are generally eliminated through
the kidneys. Urea is the largest mass
of waste metabolite produced in the liver from protein metabolism and is
generally used as a marker of renal function because it is present in large
quantities in the blood and is easily measured.
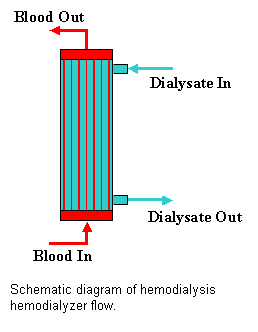
A schematic diagram of a typical
hollow-fiber dialyzer is shown in the figure at the left. The dialyzer consists of a bundle of
semi-permeable hollow fibers (tubes) surrounded by a hard plastic casing
(shell). The fibers are potted into the
casing with an impermeable glue at either end.
Fluid distribution caps are then glued into place. Blood can then flow into one fluid distribution
cap, along the interior of the fiber
(tube-side) to the exit distribution cap, and thence out of the dialyzer. Dialysate, basically distilled water with an
electrolyte and pH composition similar to that of blood plasma, flows
counter-current to the blood on the outside of the fibers (shell-side).
Chemical
species that are in the blood and not in the dialysate and that are of
sufficiently small molecular size to pass across the semi-permeable membrane, can
diffuse from the blood side to the dialysate side under a concentration
difference (potential driving force)
between the blood side concentrations (high) and dialysate side concentrations
(low). As in all potentials drive fluxes thermodynamic settings, species will move
from areas of high potential (concentration) to areas of low potential
(concentration). The overall mass
transfer equation that describes the steady-state (meaning all entering and
exiting compositions of the blood stream and the dialysate stream remain
constant with time) counter-current operation of the dialyzer is
![]()
where M i :
mass transfer rate of species i, mmol/min
K :
overall mass transfer parameter, mL/min/m2
A :
membrane area, m2
(DC
i) lm : log mean concentration difference of species
i, mmol/mL
and Kd :
dialyzer clearance = KA, mL/min.
For counter-current
flow as depicted in the figure
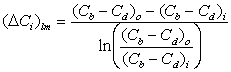
where Cb : blood concentration,
mmol/mL
Cd :
dialysate concentration, mmol/mL
subscript i :
stream inlet
subscript o :
stream outlet
The overall mass transfer coefficient,
Kd, is what is termed a “lumped parameter” that takes into consideration
the mass transfer resistances due to fluid flow on the blood side of the hollow
fiber, mass transfer resistance due to diffusion through the membrane of the
hollow fiber, and mass transfer resistance due to fluid flow on the dialysate
side of the hollow fiber. Since species
diffusion depends upon molecular weight (or more correctly, molecular size),
the overall mass transfer coefficient will be species dependent. Also, species diffusion through the membrane
will depend upon species size and permeability of the membrane to that
species. Membranes are typically
designed to limit the size of molecules that can transfer across them by
diffusive mechanisms. This is especially
important in dialysis applications where we want to remove low molecular weight
metabolic byproducts from the blood stream while retaining larger molecules
such as peptides and proteins.
In practice, the dialyzer clearance is
used to describe dialyzer performance because of the membrane area in any
particular dialyzer is fixed. Similar
comments as for the overall mass transfer coefficient apply to the dialyzer
clearance.
Hemodialysis Home End Stage Renal Disease
(ESRD) Principles
Dialyzers Modeling
Hemodialysis Therapy Demonstration History
Copyright ©
2001, John F Patzer II
All Rights
Reserved