| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |review |
 |
Up to this point,
bioaccumulation and the various related phenomena (e.g., biomagnification,
bioavailability, biotransport, etc.) have been discussed only in a
conceptual framework. Yet in practice, the ability to determine
bioaccumulation potential is a major endeavor of health regulatory agencies
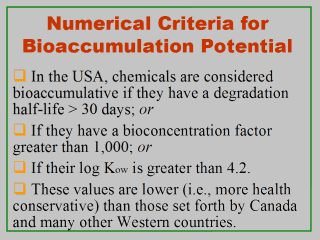
in performing risk assessments for pollutants of ecological concerns. Once a pollutant is suspected to be a potential bioaccumulant, its concentrations will be measured in ambient media (usually water) and estimated in relevant organism tissues. The bioaccumulation potential for this pollutant is then qualified by a bioconcentration factor (BCF) value, which is usually measured as the ratio of the chemicalís residues in aquatic organisms (usually fish) to its level in ambient water (Code of Federal Regulations, 2003). In the USA, pollutants are considered bioaccumulative if they have a degradation half-life > 30 days, a BCF > 1,000, or a log Kow value > 4.2 (Corl, 2001; U.S. EPA, 1999, 2000). These values are below those adopted by Canada (CEPA, 1999) and many other Western countries. Half-life is a measure of the time it takes for half of the materialís initial amount to be degraded. The chemical property Kow is an octanol-water partition coefficient, which is the ratio of a chemicalís concentration in the octanol phase to its concentration in the water phase quantifying the potential for partitioning into organic matter. Kow is inversely related to the compoundís water solubility; it represents the compoundís tendency to partition itself between an organic phase (e.g., a fish, a soil) and an aqueous phase. Its logarithmic form (log Kow) is used in models to estimate bioaccumulation factors in fish, plants, etc. |