| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |review |
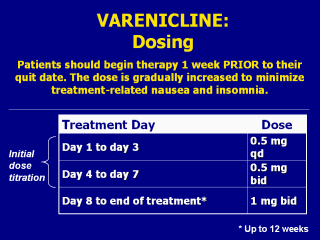 |
Treatment with varenicline should be initiated
one week BEFORE the patient stops smoking. This dosing regimen allows for
gradual titration of the dose to minimize treatment-related nausea and
insomnia. The recommended dose of varenicline is 1mg bid (taken as one 1mg
tablet in the morning and one 1mg tablet in the evening) following a 1-week
titration as follows: Treatment Day Dose Days 1–3 0.5 mg qd Days 4–7 0.5 mg bid Weeks 2–12 1 mg bid The manufacturer recommends that the dosage may be lowered temporarily or permanently for patients experiencing intolerable treatment-associated adverse effects. Patients should be treated with varenicline for 12 weeks. For patients who have successfully quit smoking at the end of 12 weeks, an additional course of 12 weeks may be appropriate to increase the likelihood of long-term abstinence. Note to instructor(s): Per the manufacturer’s prescribing information, the recommended dosage of varenicline for children, elderly patients, and individuals with impaired renal or hepatic function is as follows: Use in children Safety and effectiveness in pediatric patients have not been established; therefore, varenicline is not recommended for use in patients under 18 years of age. Dosing in elderly patients and patients with impaired hepatic function No dosage adjustment is necessary for patients with hepatic impairment. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Patients with impaired renal function No dosage adjustment is necessary for patients with mild to moderate renal impairment. For patients with severe renal impairment (estimated creatinine clearance <30 mL/min), the recommended starting dose is 0.5 mg once daily. Patients may then titrate as needed to a maximum dose of 0.5 mg twice a day. For patients with end-stage renal disease undergoing hemodialysis, a maximum dose of 0.5 mg once daily may be administered if tolerated well. Pfizer, Inc. (2006, May). Chantix Package Insert. New York, NY. Slide is used with permission, Rx for Change: Clinician-Assisted Tobacco Cessation. Copyright © 1999-2007 The Regents of the University of California, University of Southern California, and Western University of Health Sciences. All rights reserved. |