| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35|36 |37 |38 |39 |40 |41 |42 |43 |44 |45 |46 |47 |48 |49 |50 |51 |52 |53 |54 |55 |56 |57|58 |59 |review |
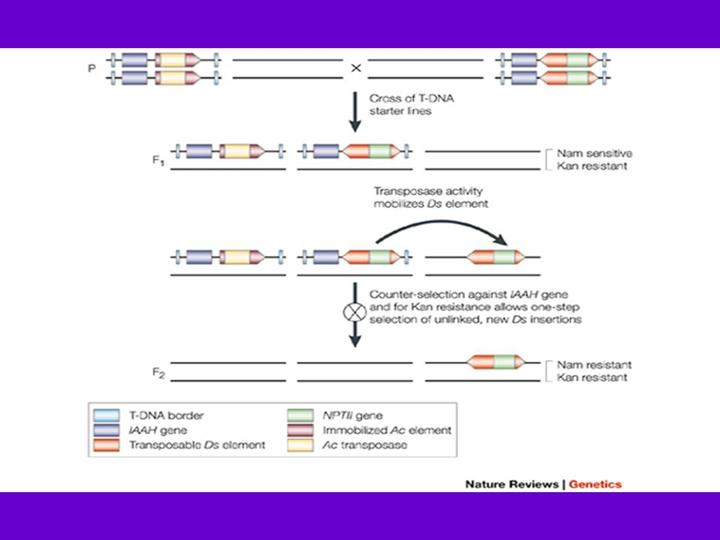 |
Transposon mutagenesis involves several steps illustrated here by the Ac/Ds transposon system developed by Sundaresan et al.86. The two starter lines are transgenic plants that carry T-DNA that contains the Ac and the Ds element, respectively. When the starter lines are crossed, Ac transposase activity allows the mobilization of the Ds element, generating genetically mosaic F1 plants. Ds elements preferentially transpose to sites that are closely linked to the donor site82, 84, so starter lines were designed that allow positive selection for the presence of a newly transposed Ds element and negative selection against the Ac and the Ds donor locus86. The immobilized Ac transposase source in the F1 is counter-selected in the F2. The Ac T-DNA vector contains an indole acetamide hydrolase (IAAH) gene that confers sensitivity to naphthalene acetamide (NAM). Ds, which carries the NPTII gene that confers kanamycin resistance, can be positively selected using kanamycin. To select against Ds elements linked to the donor Ds, the donor Ds T-DNA construct also contains the counter-selectable marker gene IAAH. This combination selects against Ac transposase, the donor Ds and Ds insertions that are linked to it, whilst selecting for unlinked Ds elements. The new transposon lines are maintained by self-pollination and are screened for phenotypes, or reporter gene expression patterns in the case of ENHANCER DETECTION and GENE TRAPPING.
|