| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |review |
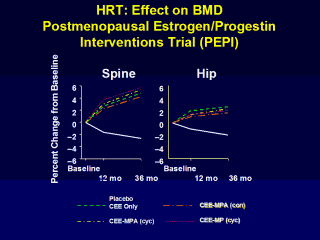 Writing Group for the PEPI Trial. JAMA, 1996;276:1389–96. |
CEE: conjugated equine estrogen; MPA:
medroxyprogesterone acetate; MP: micronized progesterone;cyc: cyclic
admin. (days 1–12 of each month); and con: continuous admin. (daily throughout the
month)
The PEPI study included 2,763 postmenopausal women. Age = 45-64 years 3-year study (randomized, double-blind, placebo-controlled) Drug = CEE only, CEE-MPA cyclic, CEE-MPA continuous, or CEE-MP cyclic Primary Endpoint = Hip and spine BMD |