 |
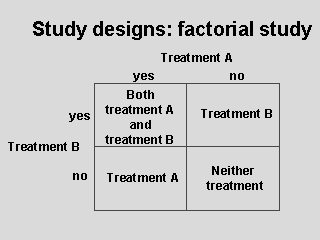
Factorial
designs allow two or more therapies to be evaluated simultaneously
in one trial. Patients are randomly assigned to one of (in this example)
four treatment groups and receive either treatment A, treatment B, both, or
neither treatment. The efficacy of each treatment regarding one or more
outcomes may be measured. The factorial design is the only method that
allows evaluation of interactions between treatments.
Many trials have used a factorial structure, including the Physician’s
Health Study, which evaluated the effect of aspirin in preventing
myocardial
infarction and the effect of beta carotene in preventing cancer. Other
studies have included combinations of three or more treatments.
|
