Repeating Sequence Copolymers (general)
Fluorescent Repeating Sequence Copolymers
Biodegradable RSC Polymers
Mercury Sensing with Lanthanide-Based Detectors
Sequence is the next great frontier of polymer chemistry. Although Nature engineers amazing variety into biopolymers by simple rearrangement of a limited set of monomers, synthetic chemists have generally been more focused on expanding the range of monomers rather than controlling their exact order in the chain. We seek to expand the understanding of sequence on polymer function beyond the biological realm for it is clear that combining sequence with the nearly unlimited set of monomers available to the synthetic chemist will result in an unprecedented expansion of macromolecular structures.
The Meyer group is interested in creating new classes of Repeating Sequence Copolymers (RSCs) and examining the dependence of properties on monomer order. A comparison of RSC with more common architectures is shown in Figure 1.
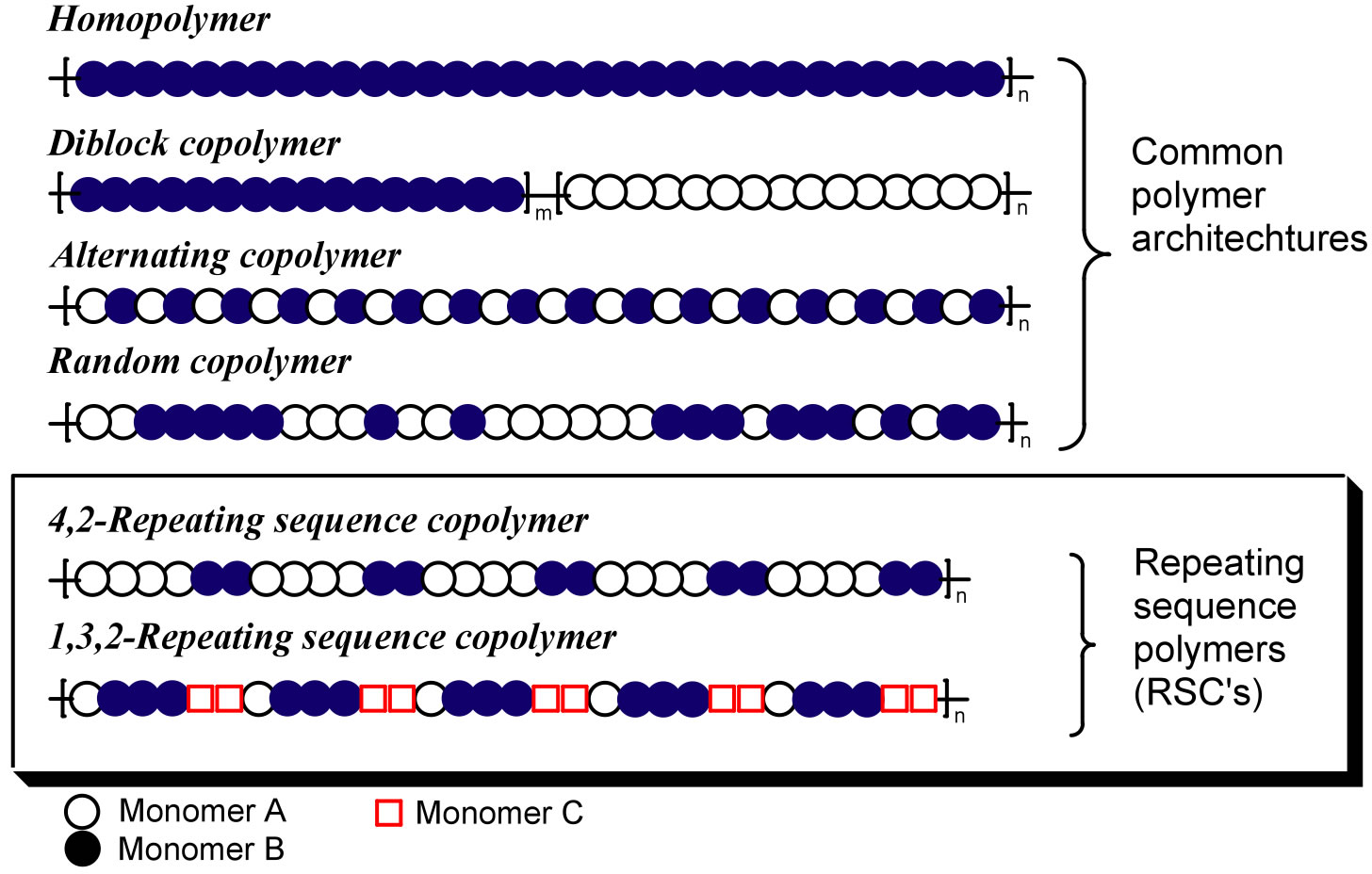
Figure 1 . Common and proposed architectures for RSCs.
Fluorescent Repeating Sequence Copolymers
Polymers that emit light are of growing interest due to their potential applications in the field of optoelectronics.1-5 Amongst this class of materials blue-light-emitting polyfluorenes (PFs) have been the focus of much attention because of the combination of thermal stability, high quantum yields, and good charge transport properties that make them highly suitable for inclusion in organic light-emitting devices, solar cells, and thin film transistors. Our group is interested in preparing sequenced polymers incorporating oligofluorene segments that will exhibit the signature PF photophysical properties while allowing for significant changes in other properties through sequence control.


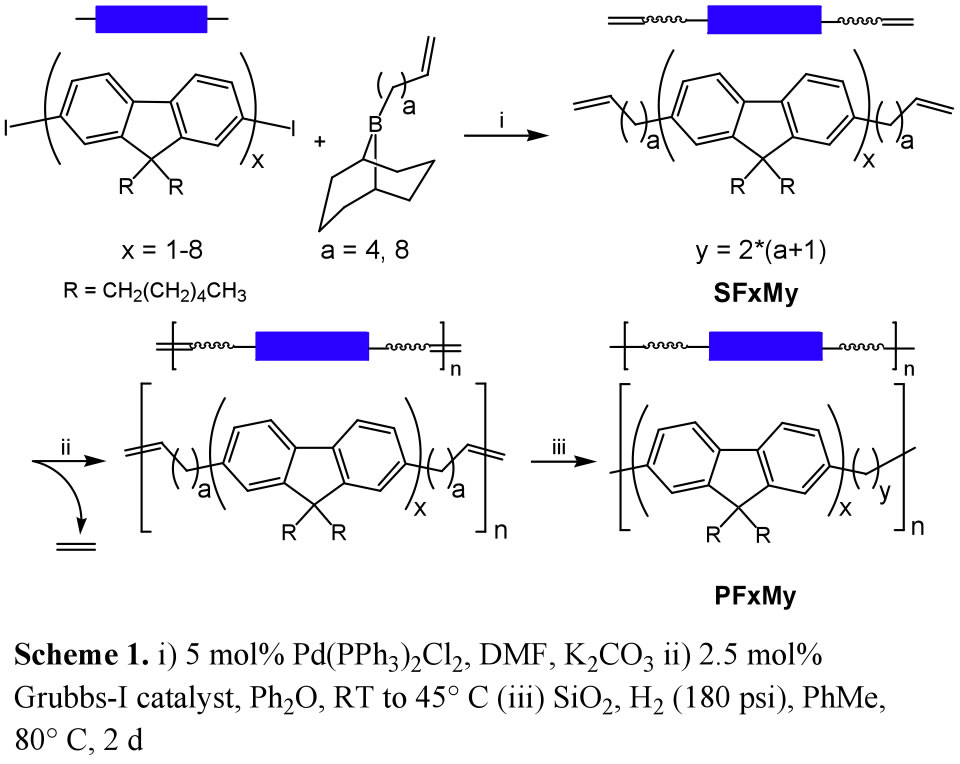
We have prepared a new class of RSCs comprising fluorene and “methylene” monomers and investigated the correlation of properties to sequence. Poly(9,9-dihexylfluorene-mb-methylene)s (PFMs) with repeat units of FxMy (x = 1-4, y = 10, 18 and x = 5-8, y = 18) were assembled by tandem metal-catalyzed acyclic diene metathesis (ADMET) polymerization/hydrogenation from
![]() -olefin bearing precursors (Scheme 1).
-olefin bearing precursors (Scheme 1).
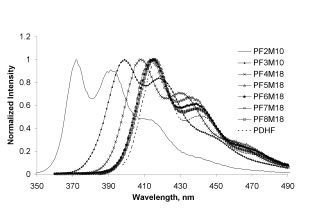
![]() Photophysical spectra exhibited the expected trends—both absorbance and emission increase in wavelength with increasing length of the fluorene block (Figure 2). The polymers were semi-crystalline with a Tm that also increased with oligofluorene length. We have recently also prepared selected members of this series with 2-ethylhexyl substituents in place of the original hexyl groups. These polymers exhibit liquid crystalline phases with transition temperatures that depend on sequence (Figure 3).
Photophysical spectra exhibited the expected trends—both absorbance and emission increase in wavelength with increasing length of the fluorene block (Figure 2). The polymers were semi-crystalline with a Tm that also increased with oligofluorene length. We have recently also prepared selected members of this series with 2-ethylhexyl substituents in place of the original hexyl groups. These polymers exhibit liquid crystalline phases with transition temperatures that depend on sequence (Figure 3).
We are also investigating the synthesis and properties other fluorescent RSCs:
.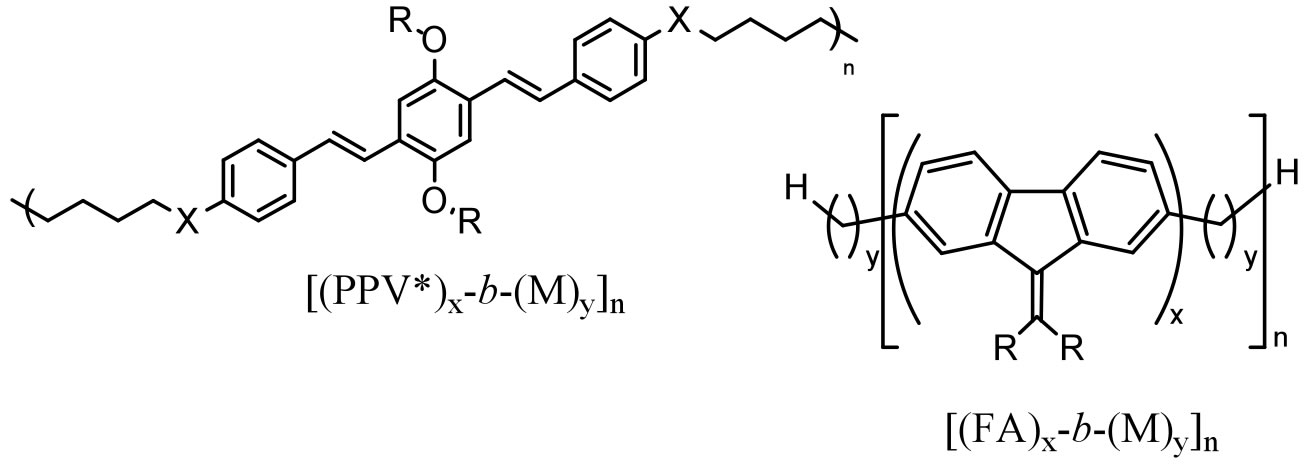
Figure 3. Fluorescent RSC polymers in preparation.
Biodegradable RSC Polymers
Poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) have been widely investigated because they are biodegradable materials that can be produced from renewable resources. The use of these polymers for in-vivo applications has been a particular focus as they are bioassimilable; hydrolysis produces the non-toxic monomers lactic and glycolic acids. Biodegradable pins, screws and sutures that are made from these materials are used clinically and it has been shown by many researchers that these polymers can be processed into 3-dimensional porous structures that can serve as scaffolds for cells targeted for tissue and bone repair.
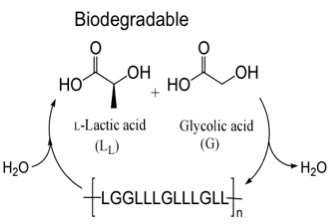


Figure 1. Poly(lactic-co-glycolic acid) is biodegradable, bioassimilable and can be prepared from renewable resources.
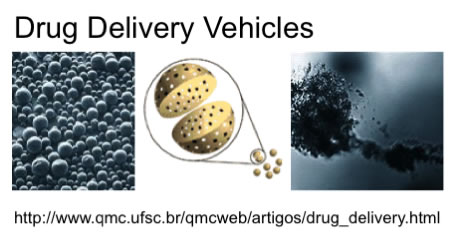

Figure 2. Current and Potential Applications of PLA and PLGA
The current and potential utility of these polymers has also inspired an interest in the microstructure of PLA and PLGA. For PLA, the homopolymer of a chiral monomer, the focus has been on tacticity. Synthetic strategies have been developed for producing atactic, isotactic, syndiotactic and heterotactic polymers. Not surprisingly mechanical, thermal and spectral properties are highly dependent on the diastereomeric relationships of the monomers. For PLGA, the main interest is sequence. In particular, since the vast majority of PLGAs reported in the literature are random copolymers, the focus has been on controlling and characterizing the ratio of monomers and average block length. Although random copolymers have been made using both stereopure and racemic lactic acid, the correlation between tacticity and properties is much less sophisticated than in the PLA system.
We can now prepare PLGA with an unprecedented level of synthetic control via the condensation polymerization of preformed segmers (oligomeric repeating units) and we are undertaking a systematic study of the correlation of the properties of the polymer with the sequence and tacticity of the polymers.
Poly(LG): alternating copolymer with L-lactide
Poly(LracG): alternating copolymer with rac-lactide
Poly(LsGLrG): alternating copolymer with exact placement of L-lactide and D-lactide
Poly(LLG): trimeric sequence with L-lactide
Poly(GGL): trimeric sequence with L-lactide
Poly(LracLG): trimeric sequence with exact placement rac-lactide and L-lactide
Poly(LLrac G):trimeric sequence with exact placement rac-lactide and L-lactide
Poly(LLGG): tetrameric sequence with L-lactide
Poly(LLLG): tetrameric sequence with L-lactide
Poly(LGGG): tetrameric sequence with L-lactide
Figure 3. Examples of PLGA RSCs
We have discovered that the chemical shifts in the 1H NMR are exquisitely sensitive to the stereochemistry of the polymer backbone. For example, the diastereotopic methylene protons for the alternating copolymer Poly(LracG) exhibit unique shifts for up to four stereocenters sequences along the chain (nearly tetrad level resolution, Figure 3). Even more complex NMR behavior is observed for longer sequences.
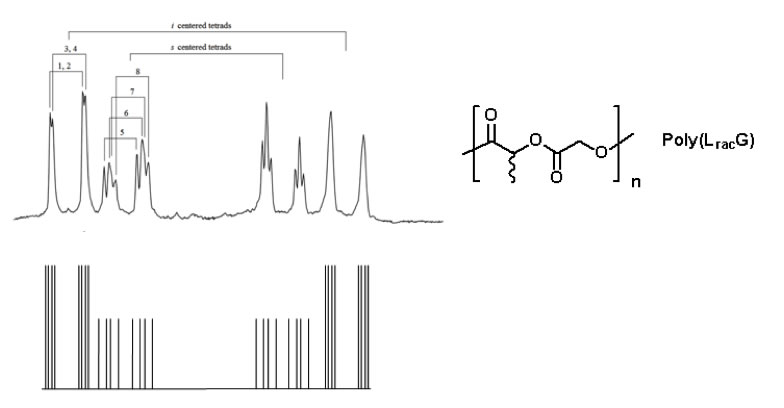
Figure 3. Illustration of the nearly tetrad resolution exhibited by the methylene protons of the glycolic monomer in the alternating copolymer. Unique chemical shifts can be resolved for 6 of the 8 possible combinations: iii, iis, isi, ssi, ssi, sis, iss and sss (top: actual spectrum in the methylene region; bottom: illustration of peak assignment for 8 nearly resolved pairs of doublets).
As we are interested in determining the suitability of PLGA RSCs for biomedical applications, we are undertaking hydrolysis studies (Figure 4). Initial results are extremely promising. We have observed, for the first time, evidence that the RSCs can exhibit an initiation time prior to hydrolysis (Figure 5). We continue to pursue these studies and to examine the role of sequence in mechanical properties.
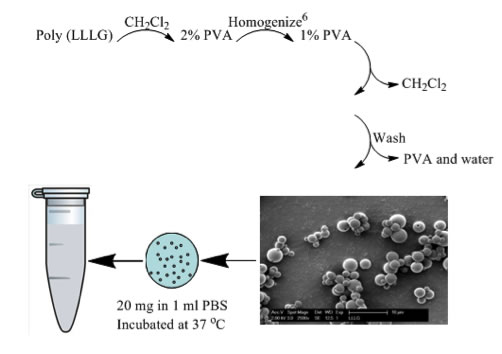
Figure 4. Hydrolysis procedure
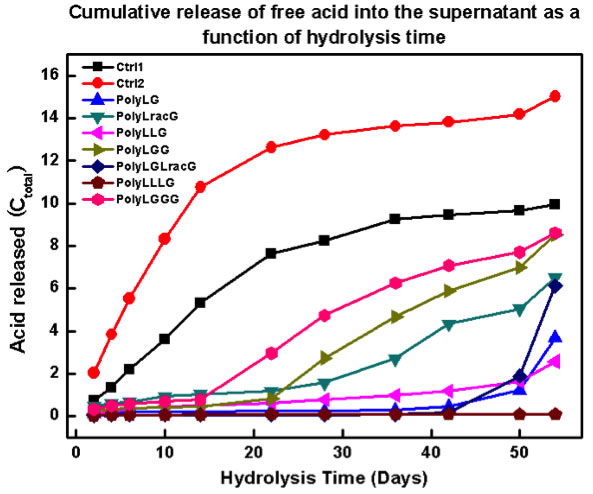
Figure 5. Plot of acid release as a function of time for several PLGA RSCs.
Mercury Sensing with Lanthanide-Based Detectors
In collaboration with the Dr. Harry Edenborn at the National Energy Technology Laboratory and Prof. Stéphane Petoud we are developing an approach to the detection of environmental mercury ions. One of the most challenging sources of mercury contamination is the burning of coal by power plants. This problem is of particular interest in Western Pennsylvania and Western Pennsylvania due to the high density of power plants (Figure 1).
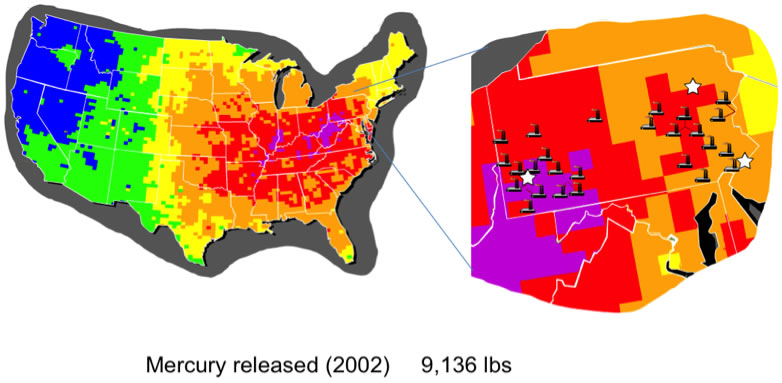
Figure 1. Mercury released in 2002.
Source: http://www.catf.us/projects/power_sector/power_plant_emissions/pollution_locator/
Our approach is modular and involves tethering ligands for both the lanthanide detector and the mercury analyte to an antenna unit. The organic antenna efficiently absorbs light and a portion of the energy is transferred to the lanthanide center, which can then luminesce. Our current system exhibits a ratiometric response to mercury. The residual fluorescence of the antenna decreases while the lanthanide luminescence decreases in the presence of mercury. The system is selective for mercury and sensitive to approximately 10-6 M concentrations of Hg2+.
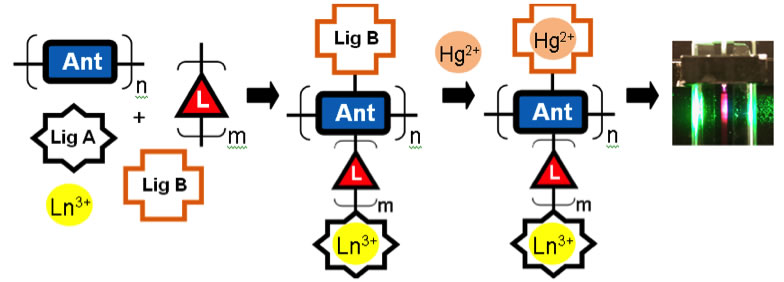
Figure 2. Schematic for the modular approach to Hg2+ sensing based on lanthanide detection.