

Next: Intracellular Calcium dynamics
Up: Channeling with Bard
Previous: Some common currents in
Most of the models that are commonly used treat the current as in
(4) but we will see that calcium is somewhat special and
requires a more complex approach.
Intracellular calcium is heavily buffered so that the concentration
tends be be very low. As a consequence the reversal potential for
calcium can not really be modeled as a fixed value. The intracellular
calcium levels are on the order of 1000 times lower than
extracellular calcium which accounts for the rather large reversal
potential for calcium. The way that many researchers model calcium is
through the constant field equation
| ![\begin{displaymath}
I_{Ca} = P_{Ca} m^ph^q \frac{4 F^2}{RT} V
\left(\frac{[Ca]_{in} e^{2VF/RT} -
[Ca]_{out}}{e^{2VF/RT}-1}\right)\end{displaymath}](img43.gif) |
(5) |
where F=96480 Colombs/mole, 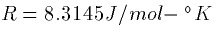 , PCa is
the permeability, and T is
the temperature in degrees Kelvin (centigrade plus 273).
, PCa is
the permeability, and T is
the temperature in degrees Kelvin (centigrade plus 273).
NOTES:
- 1 V = 1 joule/ Coulomb
- Permeability is measured in cm/sec and concentration is in
moles/liter or moles/cubic centimeter. Thus, the dimensions above are
coulombs/(sec-square centimeter) which is just current per unit area.
To get some intuition behind this expression, define
![\begin{displaymath}
E_{Ca} = \frac{RT}{2F}\ln\frac{[Ca]_{out}}{[Ca]_{in}}\end{displaymath}](img45.gif)
as is usual. Then in the above, it is clear that V=ECa makes the
current vanish so that the ``reversal potential'' is indeed the Nernst
equilibrium for calcium and linearizing about this reversal potential,
we get the slope
![\begin{displaymath}
\bar{g}_{Ca} = \frac{2P_{Ca}F^3
E_{Ca}[Ca]_{out}/(RT)^2}{[Ca]_{out}/[Ca]_{in}-1}\end{displaymath}](img46.gif)
which if you check has dimensions of conductance per unit area.
Thus, we can approximate (5) by
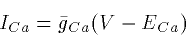
where ECa is the Nernst equilibrium potential. We will use the
two interchangeably, but keep in mind that the constant field equation
is more correct.


Next: Intracellular Calcium dynamics
Up: Channeling with Bard
Previous: Some common currents in
G. Bard Ermentrout
1/29/1998
![\begin{displaymath}
I_{Ca} = P_{Ca} m^ph^q \frac{4 F^2}{RT} V
\left(\frac{[Ca]_{in} e^{2VF/RT} -
[Ca]_{out}}{e^{2VF/RT}-1}\right)\end{displaymath}](img43.gif)
![\begin{displaymath}
I_{Ca} = P_{Ca} m^ph^q \frac{4 F^2}{RT} V
\left(\frac{[Ca]_{in} e^{2VF/RT} -
[Ca]_{out}}{e^{2VF/RT}-1}\right)\end{displaymath}](img43.gif)
![\begin{displaymath}
E_{Ca} = \frac{RT}{2F}\ln\frac{[Ca]_{out}}{[Ca]_{in}}\end{displaymath}](img45.gif)
![\begin{displaymath}
\bar{g}_{Ca} = \frac{2P_{Ca}F^3
E_{Ca}[Ca]_{out}/(RT)^2}{[Ca]_{out}/[Ca]_{in}-1}\end{displaymath}](img46.gif)
![]()