Re2O7-Catalyzed Allylic Alcohol Isomerization and Ionization Reactions
|
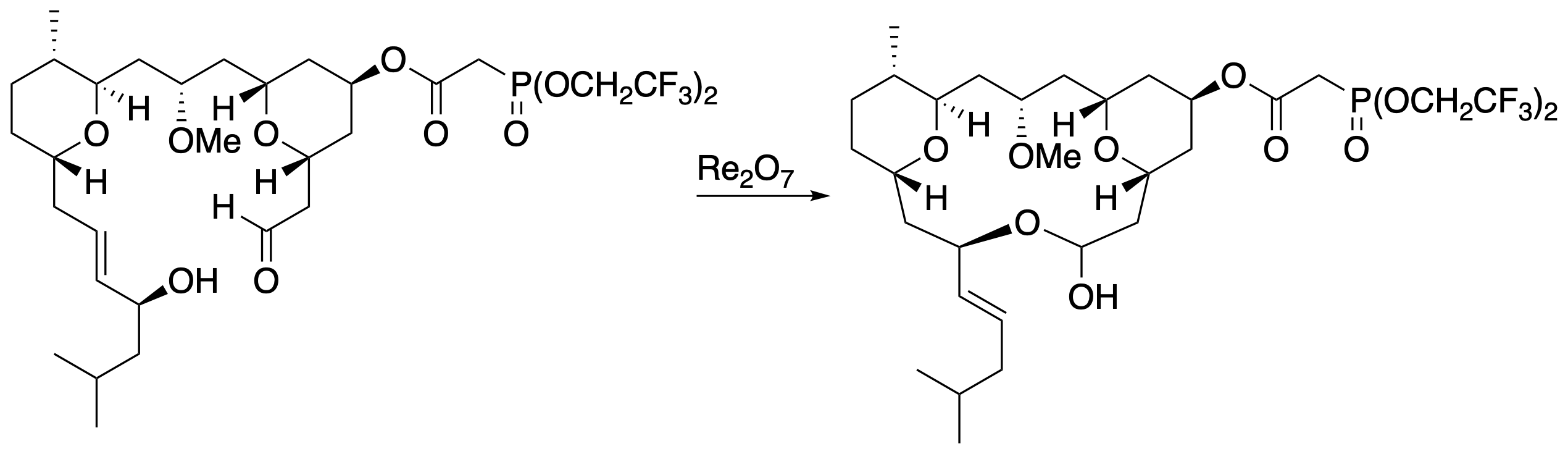
Re2O7 reacts reversibly with alcohols to form perrhenate esters. Allylic perrhenate esters can undergo transposition reactions, providing an approach to isomerizing allylic alcohols. This process is inherently non-regioselective and non-stereoselective, but product distributions can be influenced by coupling the isomerization to a thermodynamically favorable process. We became interested in this as a means of forming a macrocycle in our synthesis of leucascandrolide A, where the critical information was that only the allylic alcohol stereo- and regioisomer required for the natural product would react with the aldehyde group to form a stable macrolactol.
|
The exquisite functional group tolerance of this transformation, as evidenced by its use in a highly functionalized substrate, and the unique opportunities presented by employing thermodynamics from remote sites in a substrate to elicit stereocontrol led us to explore this process in greater detail. This involved using different stereocontrol elements and trapping groups for the transposing alcohol.
|
Vertical Divider
|
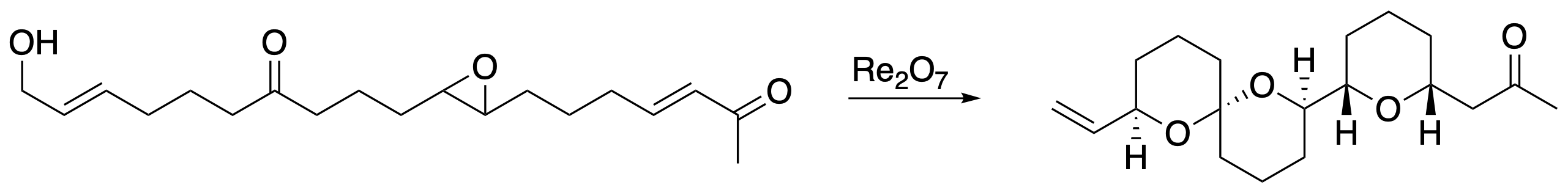
During the course of our investigations we became aware that the perrhenate ester isomerization reaction proceeded through an allylic cation intermediate. This creates opportunities to use allylic alcohols as electrophile precursors. This served as the basis for a new dehydrative approach to tetrahydropyran rings that proceeds with excellent stereocontrol, ultimately leading to a total synthesis of the natural product herboxidiene.
|
|
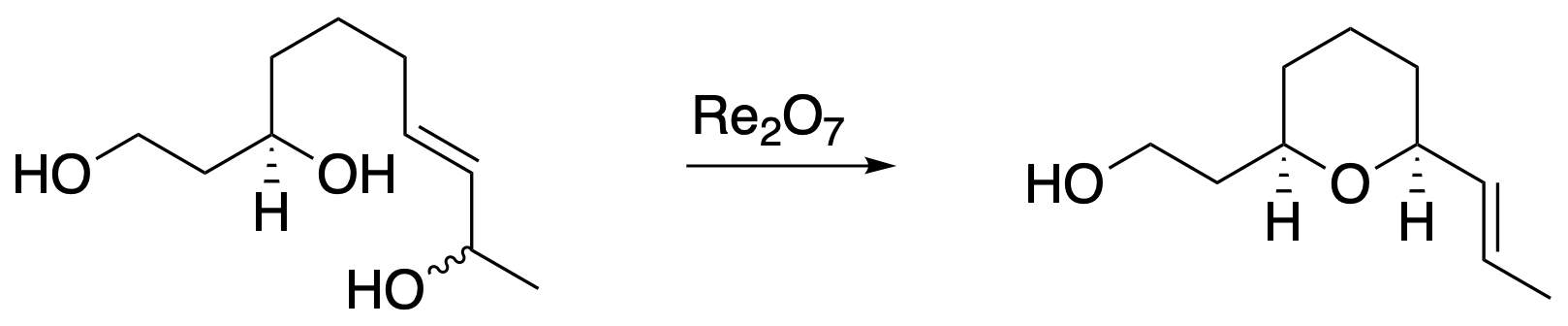
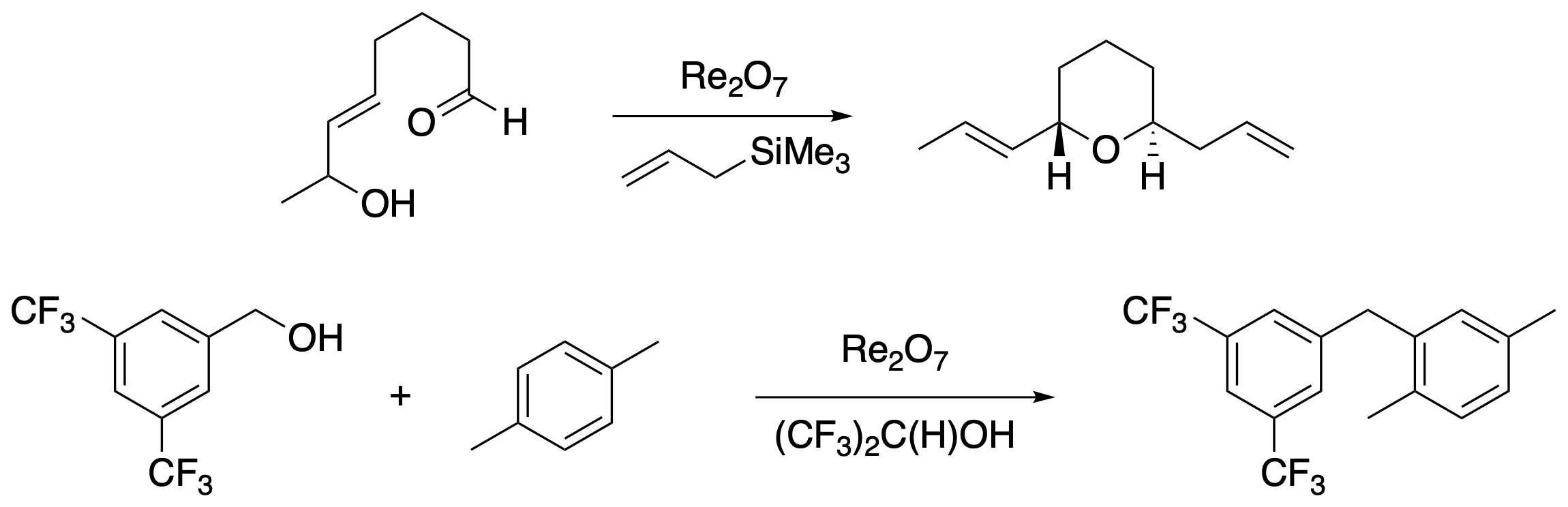
We have translated this method to bimolecular coupling processes through two strategies. Ring formation can be paired with fragment coupling through a sequence of allylic alcohol transposition cyclization into a carbonyl group, ionization to generate an oxocarbenium ion, and addition of an acid-stable nucleophile. Benzylic cations can also be generated from benzylic alcohols, and these species can engage in bimolecular Friedel-Crafts alkylation reactions, with hexafluoroisopropyl alcohol being a superior solvent. Notably, this approach shows that this system is more reactive than related approaches that use much stronger acid catalysts, thereby allowing for the generation of electron-deficient carbocations and demonstrating a competitive advantage for Re2O7 and HOReO3 as catalysts for these processes.
|
Students on this project have a blank slate to create new transformations given the ability to generate reactive intermediates under mild conditions and to exploit reaction reversibility to dictate stereochemical outcomes. The large complexity increases in many of these processes provides outstanding training in structural determination, and the functional group compatibility creates logical applications to complex molecule synthesis.