Oxidative Carbocation Formation
Oxidative carbocation formation is the longest running project in the group. The roots of the project lie in the desire to develop predictive rules for the reactivity patterns of homobenzylic ether and amide radical cations.
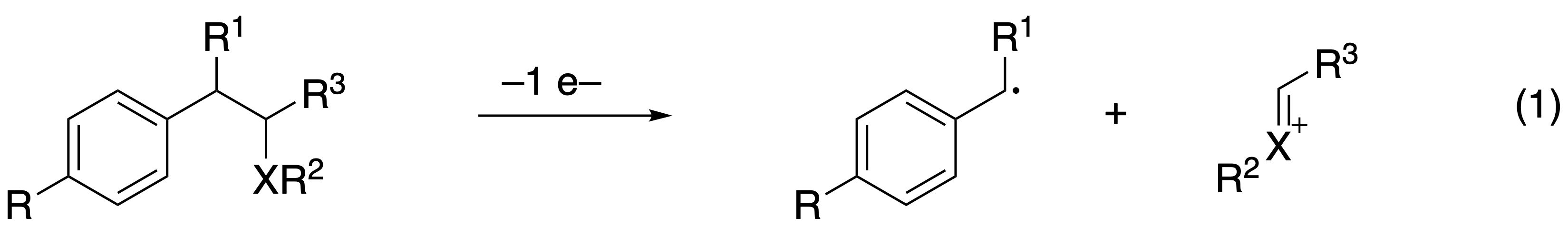
These intermediates, which can be accessed through photoinitiated electron transfer or through electron transfer to a ground state oxidant such as ceric ammonium nitrate, fragment into benzylic radicals and oxocarbenium or acyliminium ions (reaction 1). This provides access to cationic intermediates under non-acidic conditions that are compatible with a wide range of functional groups. Ring formation follows when a nucleophile is appended to the structure on either the R2 or R3 groups in the inserted graphic. This functional group compatibility allows the reactions to be employed at late stages in complex molecule synthesis. The method has thus been used in the synthesis of the natural products leucascandrolide A, theopederin D, and lactodehydrothyrsiferol, and has served as an initiator for the activation of epoxide opening cascades that provide polycyclic polyether products. Systematic structural variations have allowed for fragmentation proclivity to be predicted based on the oxidation potential of the substrate and the bond dissociation energy of the cleaving bond.
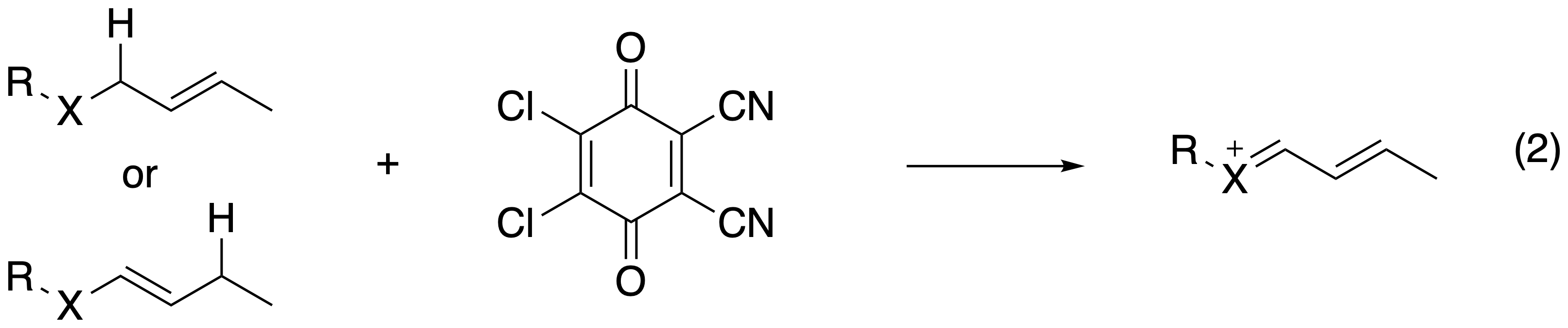
These efforts ultimately morphed into the generation of stabilized carbocations through carbon–hydrogen bond cleavage. DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) proved to be an excellent reagent for this protocol, leading to mild and operationally simple protocols to form oxocarbenium, acyliminium, and thiocarbenium ions (reaction 2).
Applying the nucleophilic trapping step from the prior studies leads to an excellent method for stereoselective heterocycle synthesis. Mechanistic nuances make these processes extremely mild and selective, again allowing for their use at late stages in complex molecule syntheses and for applications of forming carbocations at sites that would be difficult to access under classical conditions. This oxidative pathway served as the key step in the syntheses of the natural products neopeltolide, clavosolide A, bistramide A, and divergolide E. These reactions also proved to be ideal for studying the conformational preferences of highly substituted oxocarbenium and acyliminium ions.
CURRENT EFFORTS on this project focus on mitigating environmental impact by developing oxidants that can be used in substoichiometric quantities while being regenerated under green conditions, and designing new complexity-generating chemical reactions that can be accessed uniquely through oxidative carbon–hydrogen bond cleavage.
This project provides training physical organic chemistry, structural characterization, reaction optimism, and the design of projects that lead to complex target synthesis.
CURRENT EFFORTS on this project focus on mitigating environmental impact by developing oxidants that can be used in substoichiometric quantities while being regenerated under green conditions, and designing new complexity-generating chemical reactions that can be accessed uniquely through oxidative carbon–hydrogen bond cleavage.
This project provides training physical organic chemistry, structural characterization, reaction optimism, and the design of projects that lead to complex target synthesis.