Biological Studies
Our biological studies are directed in two different directions – the preparation of natural product analogs and the development of environment-specific drug delivery agents.
|
Vertical Divider
|
Proper utilization of natural products as a starting point for biological studies has several prerequisites. The natural product must show inherently interesting biological activity, the synthetic route must be sufficiently efficient to allow for material to be diverted to analog preparation, and the point of diversion must be sufficiently late in the sequence to minimize the execution of redundant reactions. The objectives for preparing analogs varies in accord with the structures that the synthetic route make available. The study can be directed toward modifying physical properties, demonstrated by the increased hydrophilicity of a hydroxylated neopeltolide analog, increasing potency, shown by the psymberin/pederin chimera that we named pedestatin, exploring conformational issues, illustrated by des-methyl herboxidiene, or increasing synthetic accessibility, highlighted by keto-bistramide.
|
Projects in this area provide an outstanding education in complex molecule synthesis and training in exploiting the design opportunities that a preparative route offers. Hypotheses regarding future research directions are generated by the results of biological assays, and these assays serve as an additional training component, even when done through collaboration.
|
Vertical Divider
|
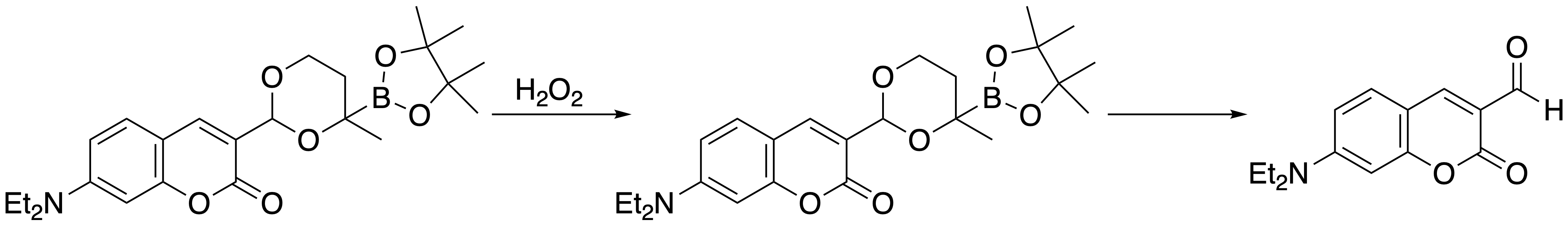
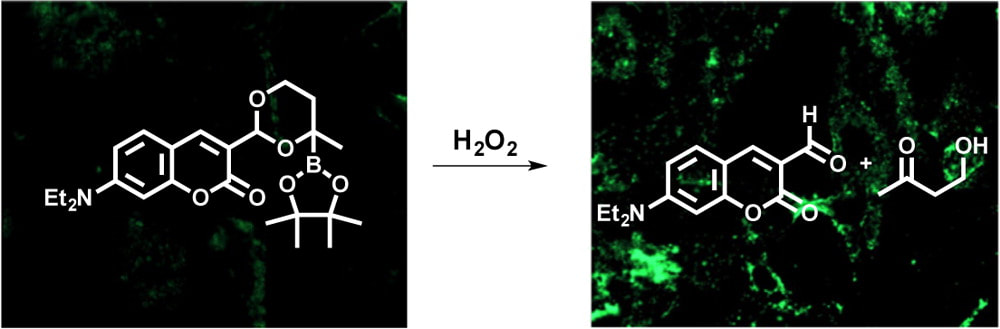
Our drug delivery efforts are based on an objective to control the distribution of a biological effector in an organism so that it is confined to a region where it will cause its response. We are working to develop agents that release drugs in the presence of elevated hydrogen peroxide. Oxidative stress, which is characteristic of numerous medical conditions, such as cancer, arthritis, viral infection, and neurodegenerative diseases, leads to the production of hydrogen peroxide. Boronates react with H2O2 to form hydroxy groups. Our design employs a-boryl ethers, which form hemiacetals upon oxidation, thereby releasing the cargo and creating a non-toxic by-product. The approach is illustrated by the release of a fluorophore from its acetal precursor. This process can be conducted in cells with endogenously generated H2O2.
|
Moving this project from fluorophore delivery to drug delivery will provide training in rational molecular design, organic synthesis, kinetic analysis and, through collaboration with the Deiters group, sophisticated biological assays.