Our laboratory seeks to develop and translate state of the art noninvasive imaging technologies to (1) improve disease diagnosis , (2) guide therapeutic strategies and (3) evaluate therapeutic efficacy. Our research emphasis is on development and application of hybrid ultrasound imaging systems that are based on a fundamental understanding of how sound and light interact with soft tissue, and are capable of assessing their mechanical, compositional, and biological characteristics. Three independent, but related, imaging technologies are under active investigation:
(1) Ultrasound elasticity imaging (UEI)/shear wave elasticity imaging (SWEI)
(2) Ultrasound Thermal Strain Imaging (TSI)
(3) Photoacoustic Imaging (PAI)/Photoacoustic molecular imaging (PMI)
(4) Super-Resolution Ultrasound Imaging
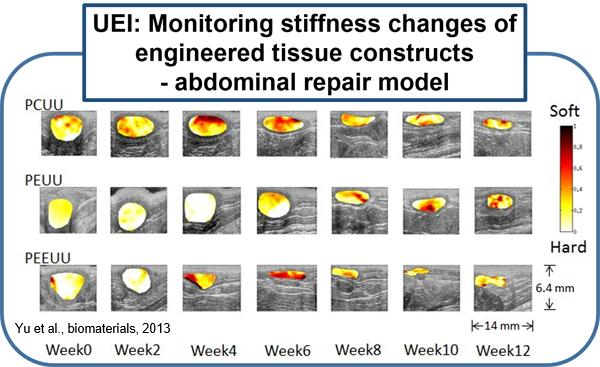
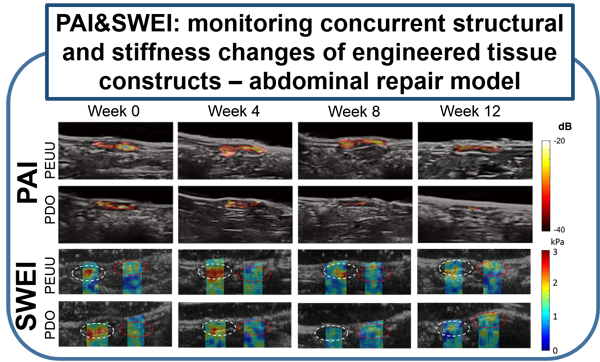
Previous attempts at noninvasive vascular elasticity imaging are limited by indirect and imprecise assessments of arterial wall motion. UEI can directly measure intramural wall strain using high resolution ultrasound speckle tracking, which results in a more precise regional assessment. Previous attempts at measuring arterial wall motion were also limited by the limited distention of arteries under physiologic pressure, and their resulting small strain. The arterial wall, however, is a highly nonlinear elastic medium. We have developed a novel "nonlinear" UEI technique that significantly increases the sensitivity of conventional UEI, and enables detection of very subtle changes in tissue stiffness that might occur at early stages of inflammation. Our nonlinear model has been validated by computer simulation as well as in vitro and ex vivo experiments using synthesized tissue phantoms and excised tissues. Preclinical in vivo studies using small animal models of various disease states are currently under active investigation or in planning stages. These disease states include atherosclerotic plaque, myocardial infarction, right ventricular failure related to pulmonary hypertension, inflammatory bowel disease, and engineered tissue constructs for abdominal repairs, vascular grafts, and cardiac patches. (Yu et al., biomaterials, 2013)
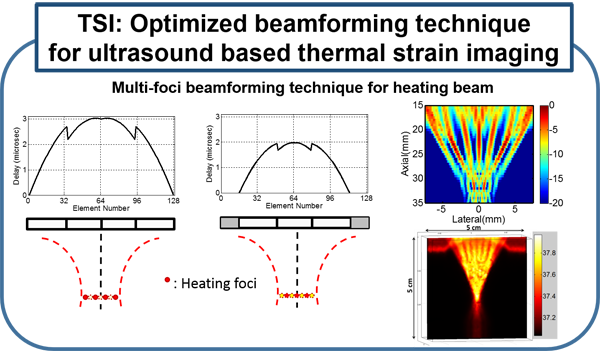
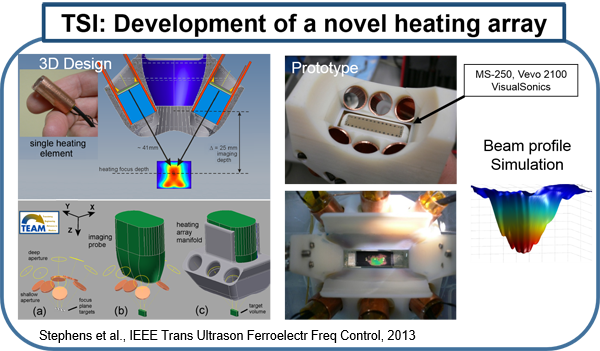
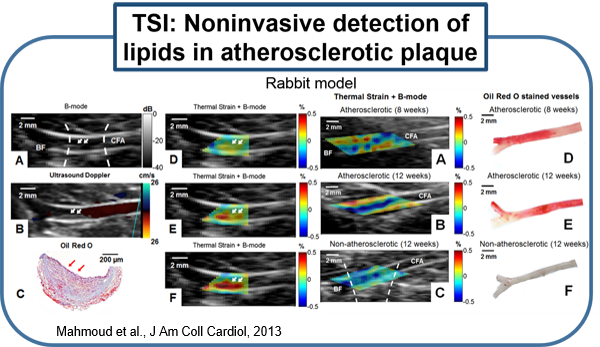
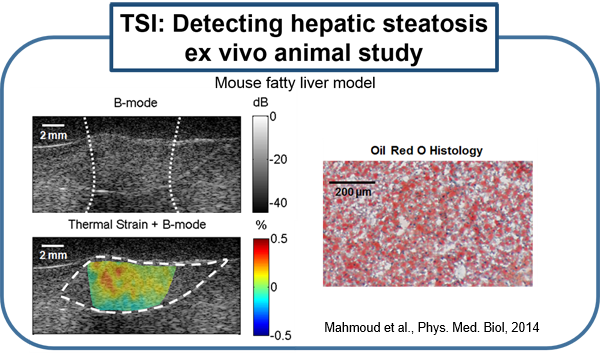
Several existing vascular imaging modalities can be used to measure the geometry of an atherosclerotic plaque, producing measurements such as luminal diameter, stenosis, wall thickness, and plaque volume. Few imaging modalities can accurately resolve plaque composition, which is an important indicator of plaque stability. We have developed ultrasound-induced thermal strain imaging (TSI) technology to detect lipid in plaque. The investigation of TSI is based on the findings that lipid-bearing tissue has a negative temperature dependence of sound speed change, while water-bearing tissue has a positive dependence. US-induced TSI uses focused ultrasound to increase the tissue temperature slightly (typically less than 1.5oC) and determines the sound speed before and after heating (rather "warming") using correlation-based speckle tracking. This system produces strong contrast between lipid-bearing and water-bearing tissue, which allows for accurate detection of the lipid-laden pool within a vulnerable plaque from surrounding water-based tissue. Translation of the technology may involve design of a hybrid ultrasound probe capable of heating tissue and imaging, preferably using an existing ultrasound scanner. A novel heating array transducer was developed and integrated into a commercial imaging ultrasound system. TSI has been validated in both in vitro and ex vivo experiments using synthesized tissue phantoms and excised tissues, including the surgical tissue samples from carotid endarterectomy and knee amputation. In vivo validation using a high fat high cholesterol rabbit model of atherosclerotic plaque has been studied. Excised human surgical tissue samples from carotid endarterectomy also has been examined. TSI to detect lipids in fatty liver is under active investigation using ob/ob mouse model. (Stephens et al., IEEE Trans Ultrason Ferroelectr Freq Control, 2013; Mahmoud et al., J Am Coll Cardiol, 2013; Mahmoud et al., Phys. Med. Biol, 2014; Nguyen et al., Ultrasound Med Biol, 2017)
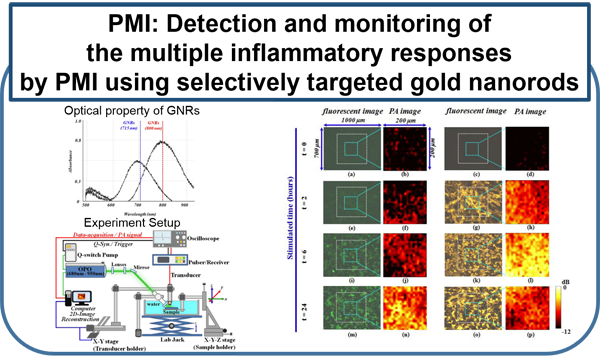
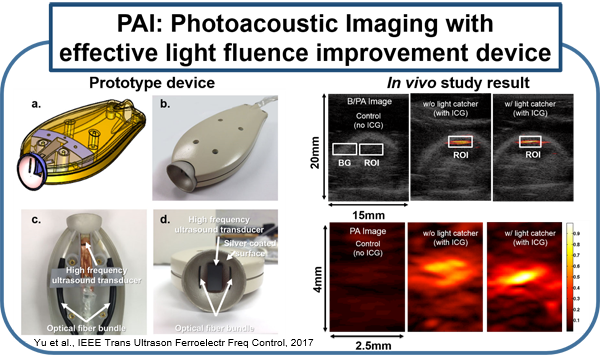
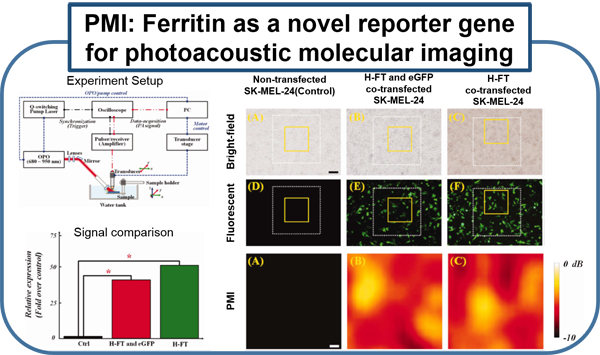
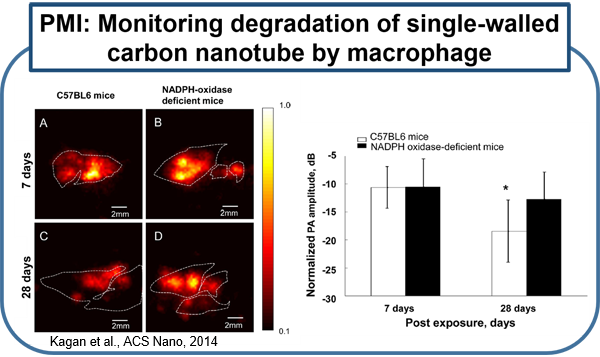
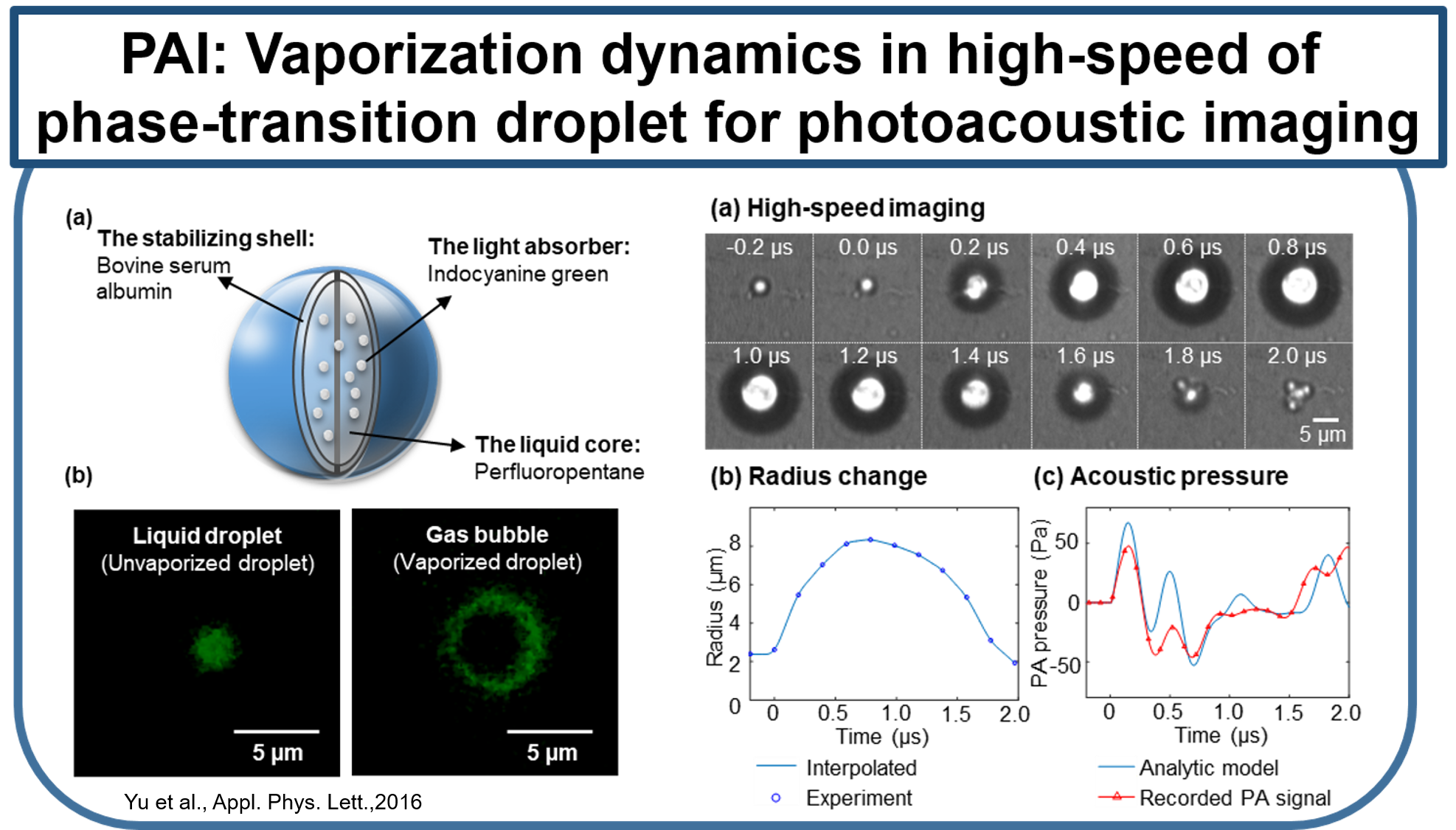
Current molecular imaging technologies such as PET, SPECT, CT, and MR, allow clinicians to identify and monitor physiological/biological changes at the cellular/molecular level, but widespread implementation is limited by 1) expense of equipment and specialized personal 2) time required for procedure and image processing and 3) risks associated with ionizing radiation and strong magnetic fields. Ultrasound is a very well-established and safe bed-side technology capable of obtaining real-time imaging of morphological features and hemodynamics. Combining conventional ultrasound imaging and laser technologies, photoacoustic (PA) molecular imaging has the potential to provide a noninvasive, real-time, bed-side molecular imaging system. Our interest in characterizing the inflammatory state of atherosclerotic plaques is based on recent advances in basic sciences that have established a fundamental link between inflammation and atherosclerotic plaque, as well as thrombotic complications of atherosclerosis. It is now known that increased expression of inflammatory markers at the molecular level predicts clinical outcomes in patients with acute coronary syndromes, independently of myocardial damage. In addition, low-grade chronic inflammation, as indicated by levels of the inflammatory marker ICAM-1, prospectively defines risk of atherosclerotic complications, thus adding to prognostic information provided by traditional risk factors. In our lab, using a mouse vascular injury model, we demonstrated that PMI using a commercial ultrasound scanner can detect gold nanoparticles bound to the vascular inflammatory biomarkers such as ICAM-1 and/or E-selectin. These principles and techniques have also successfully applied to a rat and mouse inflammatory bowel disease models. (Kagan et al., ACS Nano, 2014; Yu et al., Appl. Phys. Lett.,2016; Yu et al., IEEE Trans Ultrason Ferroelectr Freq Control, 2017)

The spatial resolution of conventional ultrasound imaging techniques is inherently limited due to the acoustic diffraction limit. Super-resolution ultrasound imaging is an emerging technology that can achieve a high spatial resolution of vasculature beyond the acoustic diffraction limit. The unprecedented spatial resolution is accomplished by combining the use of US contrast agents that enhance hyperechoic contrast, ultrafast frame rate imaging, advanced clutter filtering technique that extracts signals only from microbubbles circulating in the vessels, and novel center localization techniques that pinpoint the center of each microbubble from the extracted signals. Thus, the individual microvessels can be identified with unmatched spatial resolution, significantly outperforming conventional US approaches. In previous studies, super-resolution ultrasound imaging technology has been tested successfully in vivo for imaging microvessels in different animal model and show a great potential for diagnostic and prognostics of diseases associated with abnormal microvascular alterations. Our group has developed a deconvolution-based super-resolution imaging technology with spatio-temporal-interframe-correlation (STIC) algorithm that significantly improves the temporal resolution and mitigates the cardiac motion artifacts. The technology was successfully tested in vivo on the mouse acute kidney injury model and rabbit atherosclerotic plaque model.
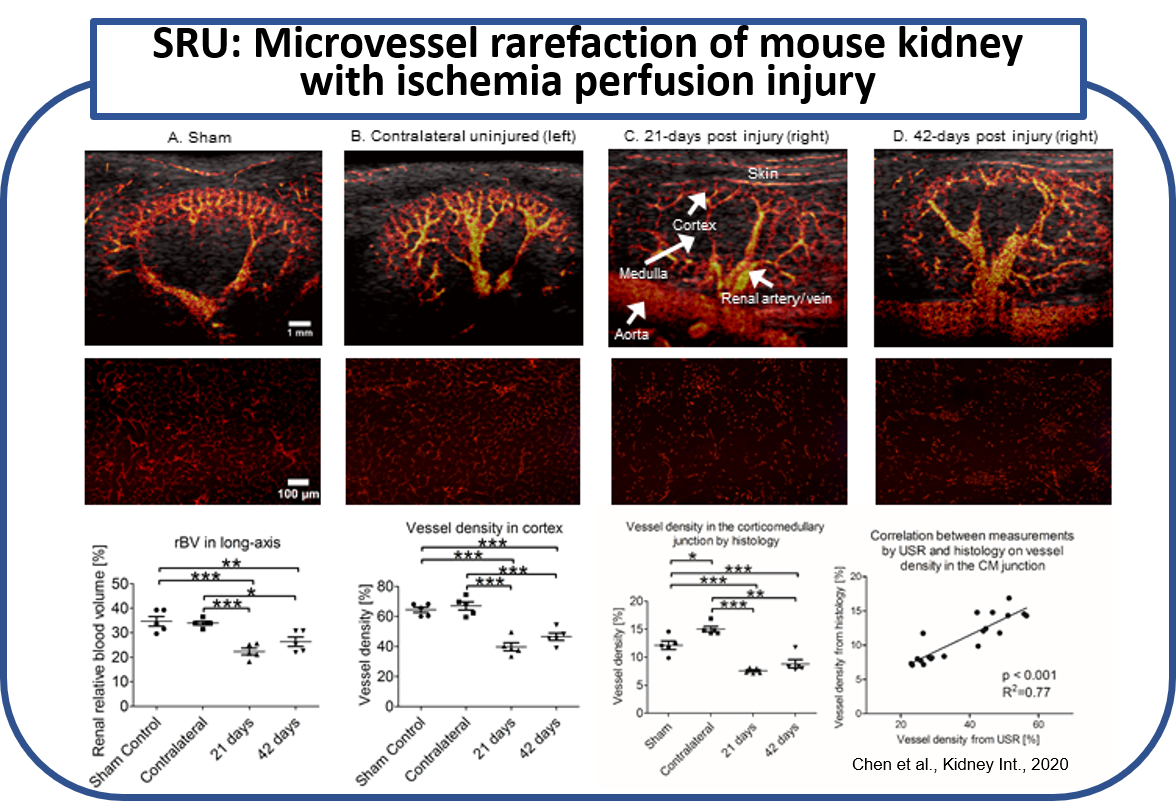
We applied this imaging technique to identify microvessels in the unilateral ischemia-reperfusion injury mouse model of AKI-to-CKD progression in vivo. Kidneys from 21 and 42 day postischemia-reperfusion injury, the contralateral uninjured kidneys, and kidneys from sham-operated mice were examined by ultrasound super-resolution and histology. Renal microvessels were successfully identified by this imaging modality with a resolution down to 32 mm. Significant reduction in kidney size, cortical thickness, relative blood volume, and microvascular density as assessed by this imaging. Tortuosity of the cortical microvasculature was also significantly increased at 42 days compared to sham. These vessel density measurements correlated significantly with CD31 immunohistochemistry (R=0.77). (Chen et al., Kidney Int., 2020)
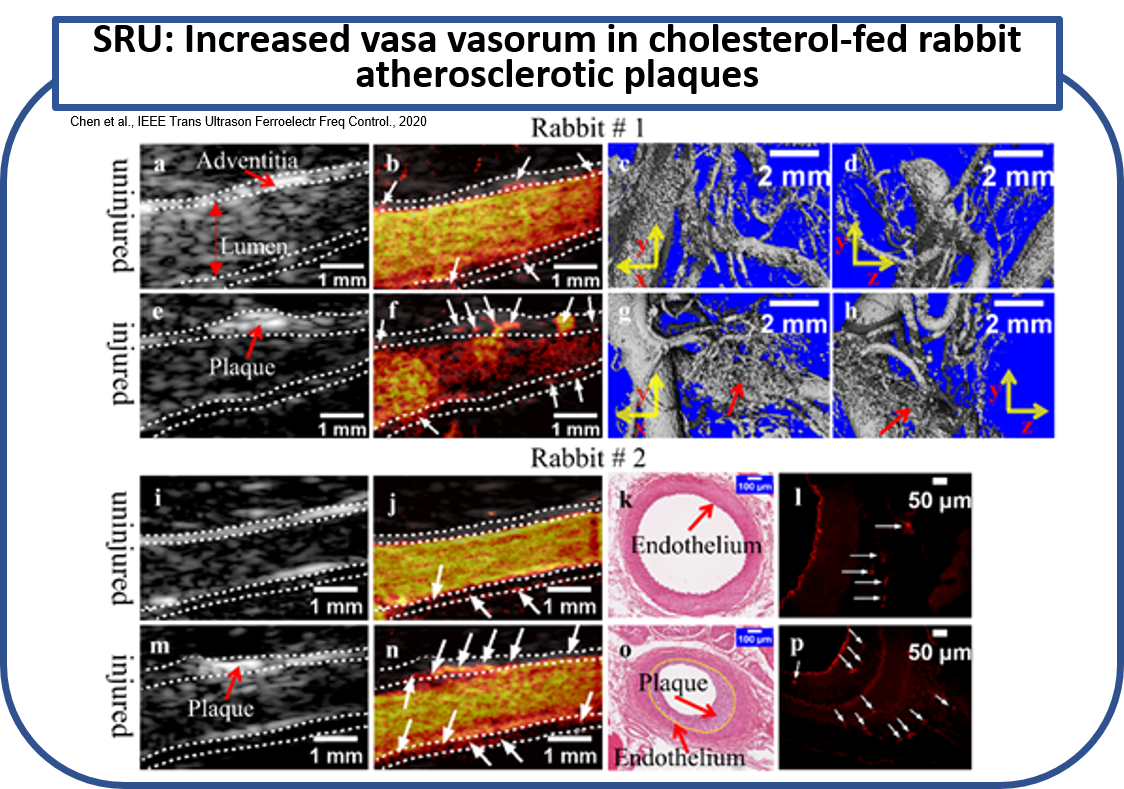
The applied the deconvolution-based super-resolution ultrasound imaging in vivo on two rabbits of a well-established AP model for identifying the VV in both injured and uninjured femoral arteries. The femoral artery and surrounding VV, as well as some other small vessels nearby, were clearly identified by super-resolution ultrasound imaging. Significant abundance of VV was found in the injured side compared to the uninjured side. The identification of VV by USR was morphologically and histopathologically verified by ex vivo μCT scan, and hematoxylin and eosin (H&E) and CD31 stain, respectively. (Chen et al., IEEE Trans Ultrason Ferroelectr Freq Control., 2020)
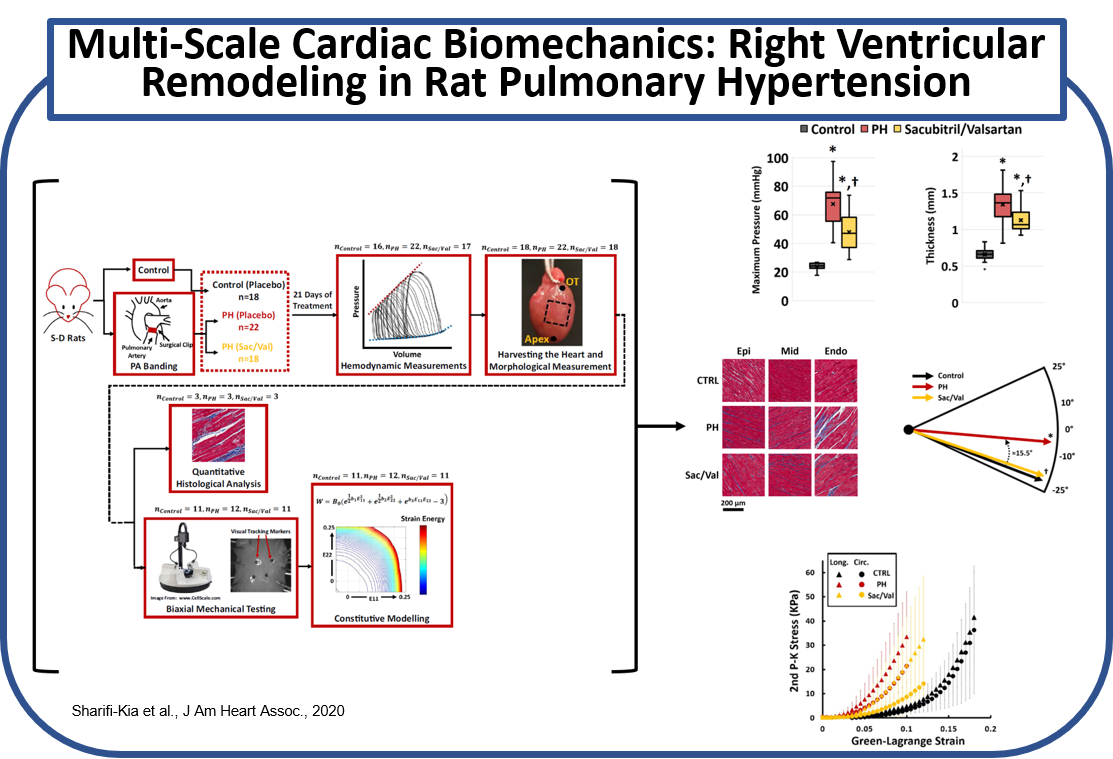
(a) Effects of Angiotensin Receptor-Neprilysin Inhibition on Right Ventricular Remodeling in Pulmonary Hypertension
Effects of angiotensin receptor-neprilysin inhibition on right ventricular (RV) remodeling in pulmonary hypertension (PH) was investigated using an animal model of PH. PH was induced via pulmonary artery banding (PAB), followed by placebo or Sacubitril/Valsartan (Sac/Val) treatment. Multi-scale biomechanical characterization of RV structure/function was performed via in-vivo hemodynamic measurements, followed by morphological measures, biaxial mechanical testing of RV free wall, quantitative transmural histology of RV collagen and myofiber architecture, and constitutive modeling of RV free wall biomechanics. Sac/Val attenuated RV remodeling in PH by reducing RV pressures, preventing hypertrophy and stiffening both at the tissue and myofiber level, in addition to preventing collagen and myofiber reorientation. (Sharifi-Kia et al., J Am Heart Assoc., 2020)
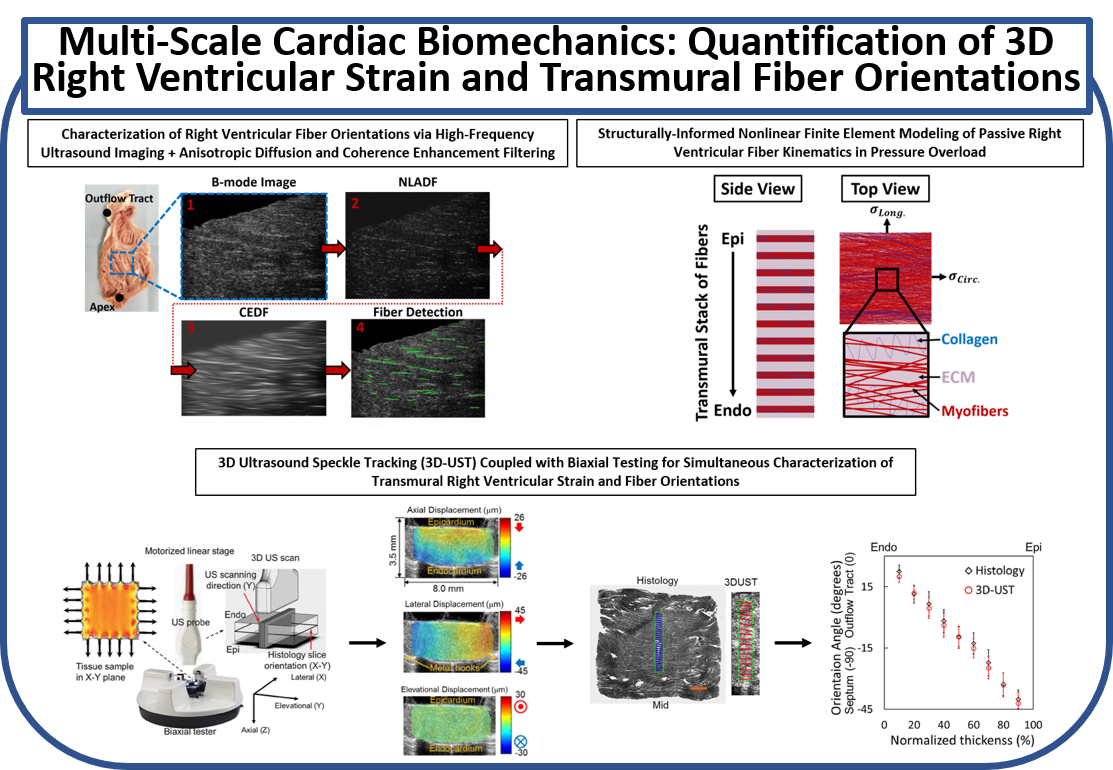
(b) Quantification of 3D Right Ventricular Strain and Transmural Fiber Orientations Using Ultrasound Imaging and Structurally-Informed Finite Element Models
A framework was developed to analyze high-frequency ultrasound images for quantification of passive myofiber reorientation under loading. Nonlinear structurally-detailed fiber embedded finite element (FE) model of the right ventricle (RV) myocardium was developed to perform parametric studies on biaxial fiber kinematics of RV free wall under pressure overload. Moreover, 3D ultrasound speckle tracking (3D-UST) was combined with biaxial measurements of RV mechanical properties for 3D full-thickness measurement of RV strains under loading. Myofiber orientations were further post-processed via constitutive modeling using 3D stress-strain measurements.
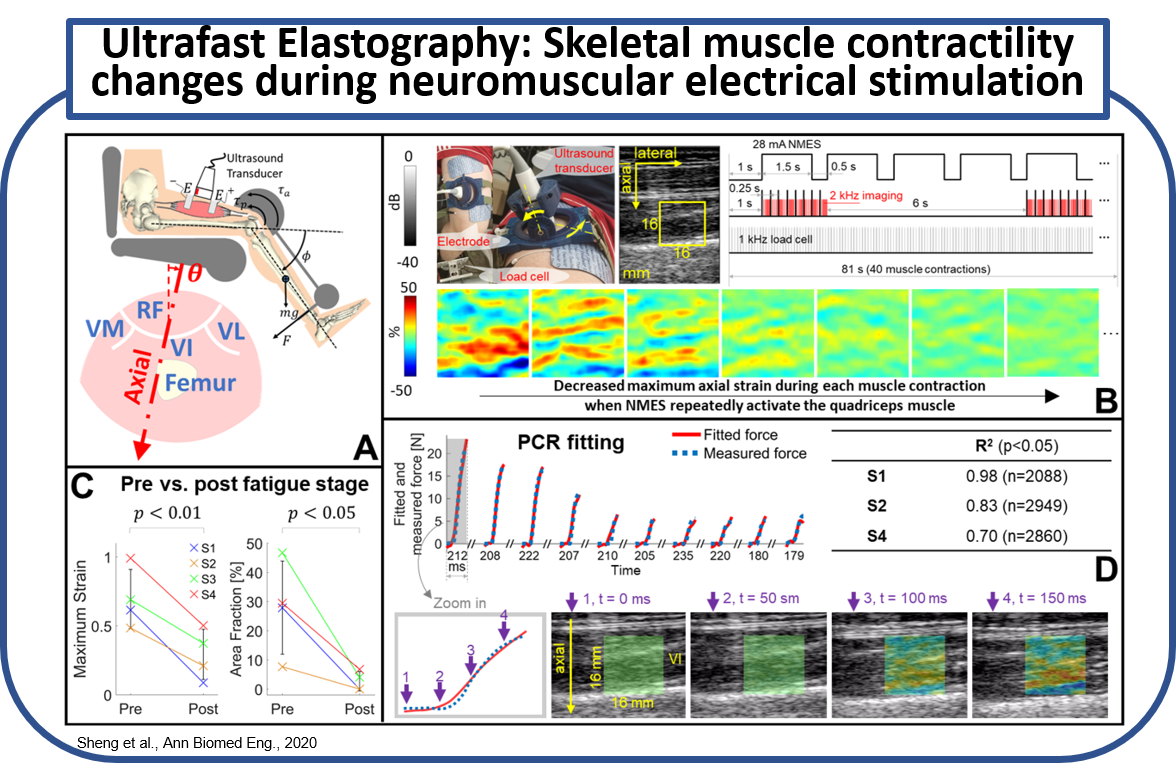
Neuromuscular electrical stimulation (NMES) can be used to artificially activate a paralyzed skeletal muscle to restore impaired limb function after spinal cord injury (SCI). However, a critical fatigue effect is noted as a rapid decline in tetanic force due to the non-physiological muscle recruitment. Subsequently, the effectiveness of NMES is quickly deteriorated if NMES dose maintains constant. A method to monitor muscle function is therefore required to adaptively modulate the NMES for an optimal dose. We proposed ulra-fast ultrasound planewave imaging with ultrasound elastography techniques to quantify the time-variant muscle contractility. (A) A sketch of isometric knee extension experiment. (B) An experiment continuously monitoring the descreased muscle contractility over repeated muscle contractions. (C) Muscle contractility compared before and after the experiment. (D) Principle component regression (PCR) to correlate the force measuremnts with the synchronized strain image sequence. (Sheng et al., Ann Biomed Eng., 2020)
Current Extramural Grants
- Development and Validation of a Multimodal Ultrasound- Based Biomarker for Myofascial Pain (NIH 1R61AT012282-01/R33AT0122821 , MPI: Kim (Wasan, Pu), 2022-2027)
- Super resolution ultrasound imaging of vasa vasorum to characterize the progression of atherosclerotic plaques and predict rupture vulnerability (NIH R01 HL157465-01A1, PI: Kim, 2022-2026)
- Prevent Unnecessary Carotid Intervention and Stroke using Noninvasive Transcutaneous Ultrasound Thermal Strain Imaging (US-TSI) (NIH R01 HL152023, PI: Kim, 2020-2026 (with NCE))
- Dual frequency intravascular ultrasound for super-resolution imaging of vasa vasorum and thin fibrous cap of vulnerable atherosclerotic plaques (R01HL178101-01, MPI: Kim (Zhou, Jiang), 2025-2029)
Previous Extramural Grants
- Closed-loop Hybrid Exoskeleton utilizing Wearable Ultrasound Imaging Sensors for Measuring Fatigue (Co-PI: Kim, CPS, NSF, 2017-2021)
- 3-D Maneuverable Feedback-Controlled Micro Swimming Drone for Biomedical Applications (Co-PI: Kim, NRI, NSF, 2016-2020)
- Advanced High Resolution Rodent Ultrasound Imaging System-Vevo3100 (PI: Kim, S10, NCRR, 2018-2019)
- Ultrasound-induced thermal strain imaging for arterial plaque characterization (PI: Kim, NIH R01, NHLBI, 2010-2015)
- Noninvasive fat quantification of liver using ultrasound thermal strain imaging (PI: Kim, NIH R21, NIBIB, 2014-16)
- A novel shear wave approach to non-invasively assess mechanical properties of liver for early diagnosis and 3D organ modeling (PI: Kim, Global Research Outreach (GRO) Program, Samsung, 2012-2015)
- Development of a novel multi-modal in-vivo imaging system for animal-to-human use (International Collation Program (University of Pittsburgh- University of Toronto- Sogang University) sponsored by the Ministry of Knowledge Economy, South Korea, 2010-2015)
- Noninvasive monitoring of tissue-engineered constructs by US elasticity imaging (PI: Kim, R21, NIBIB, 2011-2013)
- Cardiovascular inflammation function imaging by photoacoustics using gold nanorod (PI: Kim, R21, NHLBI, 2009-2012 with NCE)
- Non-invasive ultrasound elasticity imaging (UEI) in Crohn's disease (PI: Kim/Rubin, R21, NIDDK, 2009-2012 with NCE)
- Microwave induced thermal imaging (PI: Kim, R21, NIBIB, 2005-2008 with NCE)
- High-resolution ultrasound in-vivo animal imaging system-Vevo2100 (PI: Kim, S10, NCRR, 2009-2010)
Equipment
- Small Animal Ultrasonography Core: Vevo 3100
- Programmable clinical ultrasound scanner: SonixTOUCH
- High frequency small animal ultrasound scanner: Vevo2100
- Fully programmable ultrasound platform: Verasonics V1, Verasonics Vantage 128, Verasonics Vantage 256
- Nd:YAG Q-Switched tunable laser: Opotek Vibrant 532HE I