| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |review |
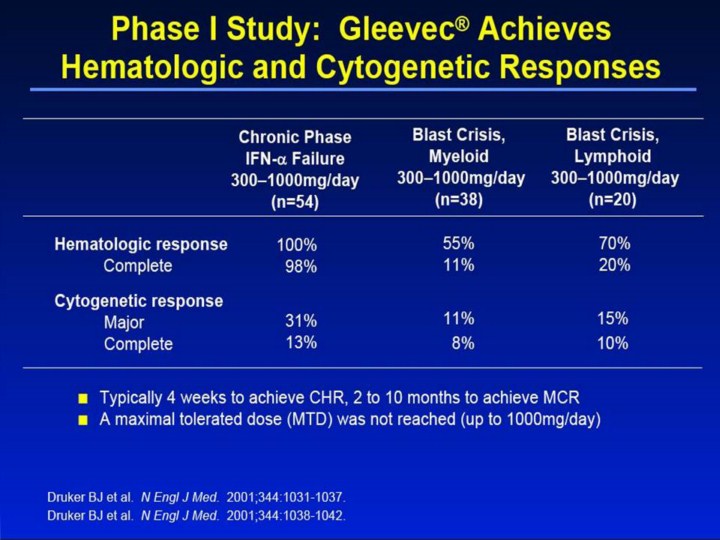 |
Phase I Study: Gleevec® Achieves Hematologic and Cytogenetic Responses1-3 Dose-finding study with treatment range of 25mg1000mg per day for patients in chronic phase and 300mg1000mg per day for patients in blast crisis. Hematologic responses in chronic phase were seen in all patients who received >140mg/day. For doses ≥300mg/day, 98% of patients achieved a CHR. Patients in chronic phase who received 3001000mg/day achieved an MCR in 31% of cases and a complete cytogenetic response in 13% of cases. MCRs were rare at doses <300mg and occurred more frequently with doses of 400mg than with doses of 300mg. A dose-response relationship was not demonstrated. Patients in chronic phase typically achieved hematologic responses within 4 weeks of initiating therapy with Gleevec. Cytogenetic responses occurred as early as 2 months after onset of treatment and continued to occur as late as 10 months after treatment initiation. Patients with lymphoid blast crisis and Ph+ ALL were grouped as lymphoid phenotype, and patients with AML and myeloid blast crisis were grouped as myeloid phenotype. The overall rate of hematologic response was 55% and 70% in patients with myeloid and lymphoid phenotypes, respectively, and the rate of MCR was 11% and 15%. High response rates were observed, but relapses were frequent among patients with advanced leukemia. The median time to relapse was 84 days for patients in myeloid blast crisis and 58 days for patients in lymphoid blast crisis. A maximal tolerated dose (MTD) was not reached, but there was evidence of an increased incidence of toxic effects at higher doses, especially at 800mg and 1000mg per day. Grade 3 or 4 myelosuppression was more frequent in patients in blast crisis than in patients in chronic phase. This difference may reflect the severely compromised bone marrow function in patients with advanced leukemia. In addition, treatment was continued throughout episodes of severe myelosuppression because of the life-threatening nature of CML in blast crisis. For safety information, please see full Prescribing Information. References 1. Druker BJ, Talpaz M, Resta D, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037. 2. Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038-1042. 3. Data on File. Novartis Pharmaceuticals Corporation, East Hanover, NJ. |