| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |review |
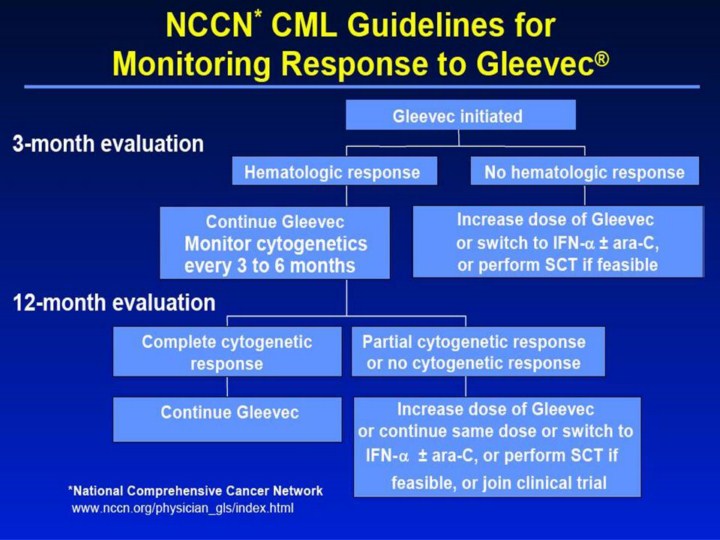 |
NCCN* CML Guidelines for Monitoring Response to Gleevec® 3-month evaluation 12-month evaluation Continue Gleevec Complete cytogenetic response Increase dose of Gleevec or continue same dose or switch to IFN-α ± ara-C, or perform SCT if Partial cytogenetic response or no cytogenetic response Continue Gleevec Monitor cytogenetics every 3 to 6 months Hematologic response Increase dose of Gleevec or switch to IFN-α ± ara-C, or perform SCT if feasible No hematologic response Gleevec initiated *National Comprehensive Cancer Network www.nccn.org/physician_gls/index.html feasible, or join clinical trial [slide 21] NCCN CML Guidelines for Monitoring Response to Gleevec®1 • The National Comprehensive Cancer Network (NCCN) has recently issued new practice guidelines for the treatment of CML. • The NCCN recommends that initial cytogenetic testing be performed 3 months after starting any drug therapy and continued at 3- to 6-month intervals. • The guidelines presented here apply to patients for whom Gleevec is prescribed. • If a hematologic response is obtained after 3 months of Gleevec as first-line therapy, treatment should be continued and cytogenetic monitoring should be performed every 3 to 6 months. • If a hematologic response is not achieved after 3 months or a complete cytogenetic response is not achieved within 12 months, increasing the dose of Gleevec or using other therapies should be considered. • According to these guidelines, a complete hematologic response is defined as complete normalization of PB counts with leukocyte count <10 x 109/L; platelets <450 x 109/L; no immature cells, such as myelocytes, promyelocytes, or blasts in PB; and no signs or symptoms of disease with disappearance of palpable splenomegaly. A partial hematologic response is defined according to the same criteria, except for the presence of immature cells; platelets <50% of the pretreatment count, but >450 x 109/L; persistent splenomegaly, but <50% of the pretreatment extent. A complete cytogenetic response is defined as no Ph+ metaphases. A partial cytogenetic response is defined as 1% to 34% Ph+ metaphases. • Also according to these guidelines: – SCT and IFN-α + ara-C have definite survival benefits whereas, despite very promising early responses, the survival benefit, long-term toxicities, and durability of response of Gleevec are unknown. – There are no available data regarding the monitoring of patients on Gleevec or the recommended treatment following response or lack of response. Reference 1. National Comprehensive Cancer Network. Practice guidelines for the treatment of CML. 2002. Available at: |