| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |review |
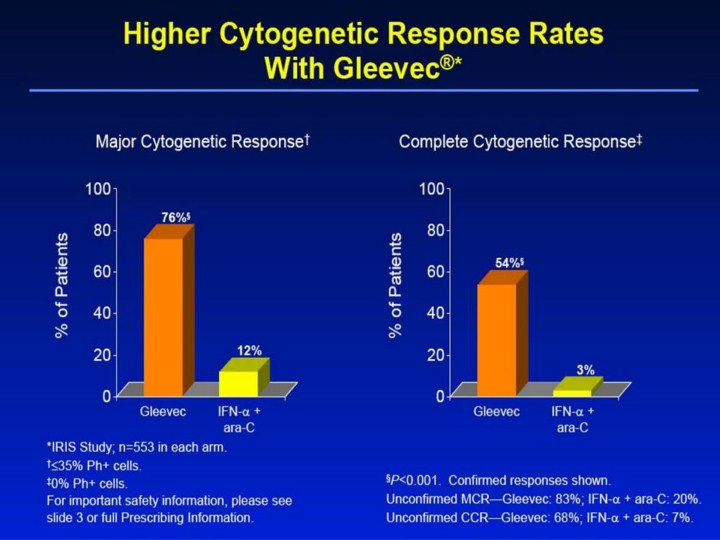 |
Higher Cytogenetic Response Rates With Gleevec®* Major Cytogenetic Response† Complete Cytogenetic Response‡ 0 20 40 60 80 100 Gleevec IFN-α + ara-C % of Patients 0 20 40 60 80 100 Gleevec IFN-α + ara-C % of Patients 76%§ 12% 54%§ 3% §P<0.001. Confirmed responses shown. Unconfirmed MCR—Gleevec: 83%; IFN-α + ara-C: 20%. Unconfirmed CCR—Gleevec: 68%; IFN-α + ara-C: 7%. *IRIS Study; n=553 in each arm. †≤35% Ph+ cells. ‡0% Ph+ cells. For important safety information, please see slide 3 or full Prescribing Information. [Slide 15] Higher Cytogenetic Response Rates With Gleevec®1,2 • The data were analyzed using Fischer’s Exact Test. • A statistically significantly higher percentage of patients achieved a major cytogenetic response (≤35% Ph+ cells) with Gleevec (76% confirmed, 83% unconfirmed) versus IFN-α + ara-C (12% confirmed, 20% unconfirmed); P<0.001. • A statistically significantly higher percentage of patients achieved a complete cytogenetic response (0% Ph+ cells) with Gleevec (54% confirmed, 68% unconfirmed) versus IFN-α + ara-C (3% confirmed, 7% unconfirmed); P<0.001. • Confirmed responses were observed on 2 occasions ≥4 weeks apart; unconfirmed responses were observed on 1 occasion. References 1. Gleevec® (imatinib mesylate) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2003. 2. Data on file. Novartis Pharmaceuticals Corporation, East Hanover, NJ. |