| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |review |
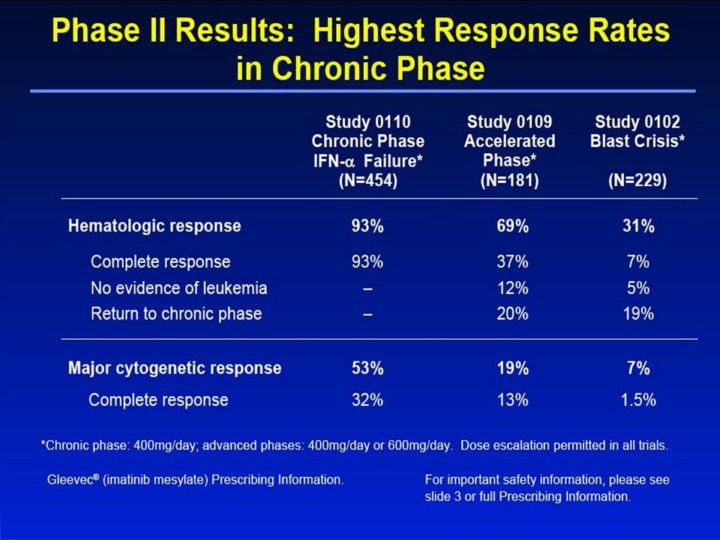 |
Phase II Results: Highest Response Rates in Chronic Phase Study 0110 Chronic Phase IFN-α Failure* (N=454) Hematologic response 93% 69% 31% Complete response 93% 37% 7% No evidence of leukemia – 12% 5% Return to chronic phase – 20% 19% Study 0109 Accelerated Phase* (N=181) Study 0102 Blast Crisis* (N=229) Major cytogenetic response 53% 19% 7% Complete response 32% 13% 1.5% Gleevec® (imatinib mesylate) Prescribing Information. For important safety information, please see slide 3 or full Prescribing Information. *Chronic phase: 400mg/day; advanced phases: 400mg/day or 600mg/day. Dose escalation permitted in all trials. [slide 10] Phase II Results: Highest Response Rates in Chronic Phase1 • These results demonstrate that there is a continuum in the response rate throughout all stages of the disease, with the highest response rates when therapy with Gleevec® is started in chronic phase. • Chronic phase patients received a dosage of 400mg/day; advanced phase patients received 400mg/day or 600mg/day (the currently recommended dosage). Dose escalation was permitted in all trials (see slide 22 or full Prescribing Information). • The overall rate of confirmed hematologic response ranged from 31% in blast crisis to 69% in patients in accelerated phase, and 93% in patients in chronic phase. • Importantly, the rate of confirmed MCR was 53% (unconfirmed 61%) in patients in chronic phase failing prior IFN-α therapy. • An unexpected finding has been a relatively high rate of confirmed MCR in advanced CML, both in patients with blast crisis (7%; unconfirmed 15%) and in patients with accelerated phase disease (19%; unconfirmed 25%). – These response rates contrast sharply with the negligible responses reported with traditional therapies in these patient populations. • Hematologic response criteria (all responses confirmed after ≥4 weeks). – Chronic phase: complete response: WBC <10 x 109/L, platelets <450 x 109/L, myelocytes + metamyelocytes <5% in PB, no blasts and promyelocytes in PB, <20% basophils in PB, no extramedullary disease – Accelerated phase and blast crisis: complete response: absolute neutrophil count ≥1.5 x109/L, platelets ≥100 x109/L, no blasts in PB, BM blasts <5%, no extramedullary disease. No evidence of leukemia: Same criteria as for CHR, but ANC ≥1 x 109/L and platelets ≥20 x 109/L. Return to chronic phase: <15% blasts in BM and PB, <30% blasts + promyelocytes in BM and PB, <20% basophils in PB, no extramedullary disease other than spleen and liver. WBC = white blood cell; PB = peripheral blood; BM = bone marrow; N/A = not applicable – Cytogenetic response criteria (all responses confirmed after ≥4 weeks except as noted) – All studies: complete response (0% Ph+ cells); major response (≤35% Ph+ cells) • Initiating treatment in chronic phase yields better responses than starting in advanced stages of CML. Reference 1. Gleevec® (imatinib mesylate) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2003. |