| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |review |
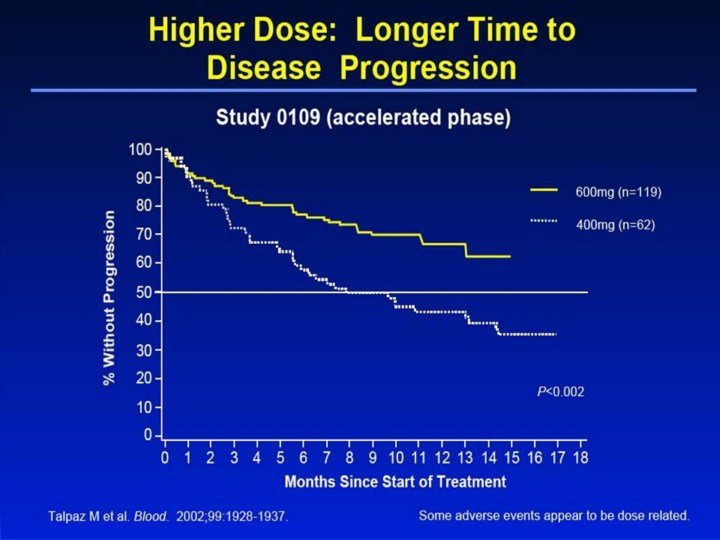 |
Higher Dose: Longer Time to Disease Progression1 • The estimated rate of patients free of disease progression (loss of response, progression to blast crisis, or death) in the accelerated phase study was significantly higher (P<0.002) with a higher dose of Gleevec®. – 44% for the 400mg dose group at 12 months – 67% for the 600mg dose group at 12 months • A variety of adverse events represent local or general fluid retention, including pleural effusion, ascites, pulmonary edema, and rapid weight gain with or without superficial edema. These events appear to be dose related, were more common in the blast crisis and accelerated phase studies (where the dose was 600mg/day), and are more common in the elderly. These events were usually managed by interrupting Gleevec treatment and with diuretics or other appropriate supportive care measures. However, a few of these events may be serious or life threatening, and one patient with blast crisis died with pleural effusion, congestive heart failure, and renal failure. Reference 1. Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928-1937. |